Abstract.
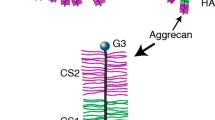
A key event in arthritis pathogenesis is the degradation of aggrecan, the major component in articular cartilage. In this work, we investigate the effects of stimulated aggrecanolysis on the morphological and nanomechanical properties of cartilage harvested from wild-type mice and aggrecanase-resistant mutant mice named “Jaffa”. The cartilages were native or were subjected to stimulated aggrecanolysis by interleukin-1\(\alpha\) (IL-1\(\alpha\)) treatment. The nanoscale morphological and mechanical properties of the sectioned cartilages were measured by using a sharp probe by atomic force microscopy (AFM). The IL-1\(\alpha\) treatment resulted in a higher nanoroughess and stiffness of the cartilage from wild-type mice. However, the same treatment did not lead to any measurable change in the nanoroughness or stiffness of the cartilage from mutant mice Jaffa. This suggests that blocking aggrecanolysis by genetic modification has created the stability in the structures and mechanical properties of the cartilage at nanoscale. The present study provides insight into the mechanism of aggrecan degradation, which can complement the examination by biochemical and histological techniques.
Graphical abstract

Similar content being viewed by others
References
H. Hedlund, E. Hedbom, D. Heinegard, S. Mengarelli-Widholm, F.P. Reinholt, O. Svensson, J. Biol. Chem. 274, 5777 (1999)
P.J. Roughley, Eur. Cells Mater. 12, 92 (2006)
L.D. Kozaci, D.J. Buttle, A.P. Hollander, Arthritis Rheum. 40, 164 (1997)
H. Stanton, S.B. Golub, F.M. Rogerson, K. Last, C.B. Little, A.J. Fosang, Nat. Protoc. 6, 388 (2011)
C.B. Little, C.T. Meeker, S.B. Golub, K.E. Lawlor, P.J. Farmer, S.M. Smith, A.J. Fosang, J. Clin. Invest. 117, 1627 (2007)
M.A. Pratta, W. Yao, C. Decicco, M.D. Tortorella, R.-Q. Liu, R.A. Copeland, R. Magolda, R.C. Newton, J.M. Trzaskos, E.C. Arner, J. Biol. Chem. 278, 45539 (2003)
H.T. Nia, L. Han, Y. Li, C. Ortiz, A. Grodzinsky, Biophys. J. 101, 2304 (2011)
H.T. Nia, I.S. Bozchalooi, Y. Li, L. Han, H.H. Hung, E. Frank, K. Youcef-Toumi, C. Ortiz, A. Grodzinsky, Biophys. J. 104, 1529 (2013)
H.T. Nia, S.J. Gauci, M. Azadi, H.H. Hung, E. Frank, A.J. Fosang, C. Ortiz, A.J. Grodzinsky, J. Biomech. 48, 162 (2015)
H.B. Wang, J.J. Wilksch, T. Lithgow, R.A. Strugnell, M.L. Gee, Soft Matter 9, 7560 (2013)
H.B. Wang, J.J. Wilksch, R.A. Strugnell, M.L. Gee, ACS Appl. Mater. Interfaces 7, 13007 (2015)
B. Lee, L. Han, E.H. Frank, S. Chubinskaya, C. Ortiz, A.J. Grodzinsky, J. Biomech. 43, 469 (2010)
M. Stolz, R. Raiteri, A.U. Daniels, M.R. VanLandingham, W. Baschong, U. Aebi, Biophys. J. 86, 3269 (2004)
E.C. Spitzner, S. Roper, M. Zerson, A. Bernstein, R. Magerle, ACS Nano 9, 5683 (2015)
M. Stolz, R. Gottardi, R. Raiteri, S. Miot, I. Martin, R. Imer, U. Staufer, A. Raducanu, M. Duggelin, W. Baschong et al., Nat. Nanotechnol. 4, 186 (2009)
J.L. Hutter, Bechhoefer, J. Rev. Sci. Instrum. 64, 1868 (1993)
S. Park, K.D. Costa, G.A. Ateshian, K.S. Hong, Proc. Inst. Mech. Eng. H 223, 339 (2009)
G.G. Bilodeau, J. Appl. Mech. 59, 519 (1992)
M.J. Rosenbluth, W.A. Lam, D.A. Fletcher, Biophys. J. 90, 2994 (2006)
M. Loparic, D. Wirz, A.U. Daniels, R. Raiteri, M.R. VanLandingham, G. Guex, I. Martin, U. Aebi, M. Stolz, Biophys. J. 98, 2731 (2010)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Uddin, M.H., Wang, H., Rogerson, F.M. et al. Effects of stimulated aggrecanolysis on nanoscale morphological and mechanical properties of wild-type and aggrecanase-resistant mutant mice cartilages. Eur. Phys. J. E 40, 72 (2017). https://doi.org/10.1140/epje/i2017-11561-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epje/i2017-11561-1