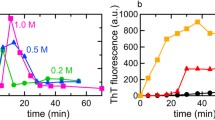
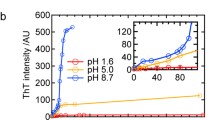
Abstract.
The analysis of amyloidogenic systems reveals the appearance of distinct states of aggregation for amyloid fibrils. For different proteins and under specific experimental conditions, amyloid spherulites are recognized as a significant component occurring in several protein model systems used for in vitro fibrillation studies. In this work we have developed an approach to characterize solutions containing a mixture of amyloid spherulites and individual fibrils. Using bovine insulin as the model system, sedimentation kinetics for the amyloid aggregates were followed using a combination of UV-Vis spectroscopy and cross-polarized optical microscopy. Spherulites were identified as the species undergoing sedimentation. A simple mathematical approach allows the description of the kinetics in terms of decay time/rate distribution. Moreover, based on the sedimentation kinetics, a rough estimate of the balance between amyloid spherulites and individual fibrils can be provided. Fitting the experimental data with the proposed physico-chemical approach shows self-consistent results in reasonable agreement with quantitative imaging analysis previously reported. Our results provide new physical insights into the analysis of amyloidogenic systems, providing a method to characterize the heterogeneous distribution of amyloid spherulites and simultaneously distinguish spherulites and free fibril populations. Importantly, the method can be generally applied to the characterization of polydisperse solutions containing optically traceable spherical particles in the micrometric range.
Similar content being viewed by others
References
F. Chiti, P. Webster, N. Taddei, A. Clark, M. Stefani, G. Ramponi, C.M. Dobson, Proc. Natl. Acad. Sci. U.S.A. 96, 3590 (1999)
M. Vendruscolo, J. Zurdo, C.E. McPhee, C.M. Dobson, Phil. Trans. R. Soc. London, Ser. A 361, 1205 (2003)
C.M. Dobson, Semin. Cell Dev. Biol. 15, 3 (2004)
M. Stefani, Biochim. Biophys. Acta 1739, 5 (2004)
V.N. Uversky, A.L. Fink, Biochim. Biophys. Acta 1698, 131 (2004)
J. Brange, Physical stability of proteins, in Pharmaceutical Formulation Development of Peptides and Proteins, edited by S. Frokjaer, L. Hovgaard (Taylor Francis, London, 2000)
M.R. Nilsson, Methods 34, 151 (2004)
V. Militello, V. Vetri, M. Leone, Biophys. Chem. 105, 133 (2003)
V. Militello, C. Casarino, A. Emanuele, A. Giostra, F. Pullara, M. Leone, Biophys. Chem. 107, 175 (2004)
V. Vetri, V. Militello, Biophys. Chem. 113, 83 (2005)
V. Vetri, C. Canale, A. Relini, F. Librizzi, V. Militello, A. Gliozzi, M. Leone, Biophys. Chem. 125, 184 (2007)
F. Oosawa, S. Asakura, Thermodynamics of the Polymerization of Proteins (Academic Press, New York, 1975)
F. Ferrone, Methods Enzymol. 309, 256 (1999)
S.B. Padrick, A.D. Miranker, Biochemistry 41, 4694 (2002)
W.F. Xue, S.W. Homans, S.E. Radford, Proc. Natl. Acad. Sci. U.S.A. 105, 8926 (2008)
A. Loksztejn, W. Dzwolak, Biochemistry 48, 4846 (2009)
M. Fändrich, J. Meinhardt, N. Grigorieff, Prion 3, 89 (2009)
J. Meinhardt, C. Sachse, P. Hortschansky, N. Grigorieff, M. Fändrich, J. Mol. Biol. 386, 869 (2009)
A.T. Petkova, R.D. Leapman, Z. Guo, W.M. Yau, M.P. Mattsona, R. Tycko, Science 307, 262 (2005)
J.L. Jiménez, G. Tennent, M. Pepys, H.R. Saibil, J. Mol. Biol. 311, 241 (2001)
R.A. Crowther, M. Goedert, J. Struct. Biol. 130, 271 (2000)
B. Seilheimer, B. Bohrmann, L. Bondolfi, F. Müller, D. Stüber, H. Döbeli, J. Struct. Biol. 119, 59 (1997)
M. Manno, E.F. Craparo, A. Podestà, D. Bulone, R. Carrotta, V. Martorana, G. Tiana, P.L. San Biagio, J. Mol. Biol. 366, 258 (2007)
V. Foderà, M. van deWeert, B. Vestergaard, Soft Matter 6, 4413 (2010)
M.R. Krebs, C.E. Macphee, A.F. Miller, I.E. Dunlop, C.M. Dobson, A.M. Donald, Proc. Natl. Acad. Sci. U.S.A. 101, 14420 (2004)
S.G. Bolder, H. Hendrick, L.M.C. Sagis, E. van der Linden, J. Agric. Food Chem. 54, 4229 (2006)
S.S. Rogers, M.R.H. Krebs, E.H.C. Bromley, E. van der Linden, A.M. Donald, Biophys. J. 90, 1043 (2006)
M.R. Krebs, E.H. Bromley, S.S. Rogers, A.M. Donald, Biophys J. 88, 2013 (2005)
C. Exley, E. House, J.F. Collingwood, M.R. Davidson, D. Cannon, A.M. Donald, J. Alzheimers Dis. 20, 1159 (2010)
J. Juárez, P. Taboada, S. Goy-López, A. Cambón, M.B. Madec, S.G. Yeates, V. Mosquera, J. Phys. Chem. B 113, 12391 (2009)
K.R. Domike, A.M. Donald, Biomacromolecules 8, 3930 (2007)
Y.F. Mok, G.J. Howlett, Meth. Enzymol. 413, 199 (2006)
V. Foderà, S. Cataldo, F. Librizzi, B. Pignataro, P. Spiccia, M. Leone, J. Phys. Chem. B 113, 10830 (2009)
V. Foderà, M. Groenning, V. Vetri, F. Librizzi, S. Spagnolo, C. Cornett, L. Olsen, M. van de Weert, M. Leone, J. Phys. Chem. B 112, 15174 (2008)
R.R. Porter, Biochem. J. 53, 320 (1953)
M. Groenning, L. Olsen, M. van de Weert, J.M. Flink, S. Frokjaer, F.S. Jorgensen, J. Struct. Biol. 158, 358 (2007)
C.P. Jaroniec, C.E. MacPhee, N.S. Astrof, C.M. Dobson, R.G. Griffin, Proc. Natl. Acad. Sci. U.S.A. 99, 16748 (2002)
M.R. Krebs, E.H. Bromley, A.M. Donald, J. Struct. Biol. 149, 30 (2005)
R. Jansen, W. Dzwolak, R. Winter, Biophys J. 88, 1344 (2005)
A. Ahmad, V.N. Uversky, D. Hong, A.L. Fink, J. Biol. Chem. 280, 42669 (2005)
S. Grudzielanek, V. Smirnovas, R. Winter, J. Mol. Biol. 356, 497 (2006)
L.L. Minkov, E.V. Pikushchak, J.G. Dueck, Thermophys. Aeromech. 6, 77 (2009)
M.N. Berberan-Santos, E.N. Bodunov, B. Valeur, Chem. Phys. 317, 57 (2005)
M.N. Berberan-Santos, Chem. Phys. Lett. 460, 146 (2008)
L. Whitehead, R. Whitehead, Am. J. Phys. 77, 173 (2009)
D. Cannon, A.M. Donald, in preparation
V. Foderà, F. Librizzi, M. Groenning, M. van de Weert, M. Leone, J. Phys. Chem. B 112, 3853 (2008)
J. Hofrichter, J. Mol. Biol. 189, 553 (1986)
P. Hammarstrom, X. Jiang, S. Deechongkit, J.W. Kelly, Biochemistry 40, 11453 (2001)
H.A. Lashuel, Z. Lai, J.W. Kelly, Biochemistry 37, 17851 (1998)
H.A. Lashuel, D.M. Hartley, D. Balakhaneh, A. Aggarwal, S. Teichberg, D.J. Callaway, J. Biol. Chem. 277, 42881 (2002)
E. Acebo, M. Mayorga, J.F. Val-Bernal, For. Pathol. 31, 8 (1999)
M.D. Griffin, M.L. Mok, L.M. Wilson, C.L. Pham, L.J. Waddington, M.A. Perugini, G.J. Howlett, J. Mol. Biol. 375, 240 (2008)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Foderà, V., Donald, A.M. Tracking the heterogeneous distribution of amyloid spherulites and their population balance with free fibrils. Eur. Phys. J. E 33, 273–282 (2010). https://doi.org/10.1140/epje/i2010-10665-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epje/i2010-10665-4