Abstract
We discuss peculiar features of electron scattering on the N2 molecule and the N2+ ion, that are important for modeling plasmas, Earth’s and other planets’ atmospheres. These features are, among others: the resonant enhancement of the vibrational excitation in the region of the shape resonance around 2.4 eV, the resonant character of some of electronic excitation channels (and high values of these cross sections, both for triplet and singlet states), high cross section for the dissociation into neutrals, high cross sections for elastic scattering (and electronic transitions) on metastable states. For the N2+ ion we discuss both dissociation and the dissociative ionization, leading to the formation of atoms in excited states, and dissociative recombination which depends strongly on the initial vibrational state of the ion. We conclude that the theory became an indispensable completion of experiments, predicting many of partial cross sections and their physical features. We hope that the data presented will serve to improve models of nitrogen plasmas and atmospheres.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen is important gas not only for Earth’s atmosphere. Phenomena, such as electrical strikes in upper atmosphere [1], optical properties of exo-planetary atmospheres [2], re-entry of space shuttles [3], microwave torch discharges [4], etc., require detailed knowledge of electron scattering phenomena on N2 and related species. At early stage of laser technologies the nitrogen discharges were used as source of high-power (MW), short (ns), and ultraviolet radiation [5]. DC and microwave discharges in N2 at atmospheric pressure find numerous technical and chemical applications [6]. Renewed interest also comes from the discoveries of the presence of nitrogen in the atmospheres of Titan, Pluto and rocky exoplanets [7].
Particularly complex is modeling of N2 (and air) industrial plasmas. In a recent evaluation [8] of the population of atomic and molecular (excited, dissociated, ionized) species in the nitrogen plasma torch as many as 13 electronically excited states of N2 were included, but the results are still not satisfactory. A peculiarity of N2 electrical discharges and plasmas is the importance of transitions from electronically and vibrationally excited states. In the visible and near-UV range optical emission spectra these are transitions B 3Πg → A 3Σu+ (“1st positive system”) and C 3Πu → B 3Πg, (“2nd positive system”) [9]. Similarly important are transitions initiating from vibrationally excited levels, see modeling by Colonna et al. [10].
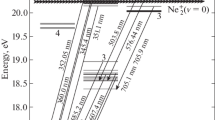
In an experimental study [11] of the far ultraviolet emission (120–170 nm) from N2 molecules excited by 30–200 eV electrons, it was shown that the Lyman–Birge–Hopfield (LBH) band is largely determined by cascading between electronic transitions, see Fig. 1. Not only modeling but even a mere explanation of observed emission features requires knowledge of cross sections for vibrational and electronic transitions between many states. Furthermore: These data are key for interpreting space borne and astrophysical observations.
Experimental studies of the UV glow in the Lyman–Birge–Hopfield band at far radial distances from the electron beam, at 100 eV electron impact energy and 5 × 10–5 Torr N2 pressure. The explanation of the observed spectra required assuming two processes: a fast (50 μs) direct de-excitation of the a 1Πg state and a slow (~ 5000 μs) cascade-induced de-excitation of the two states (a´1Σ−u and w 1Δu) → a 1Πg state → X 1Σ+g). From Ref. [11], reproduced with permission
In this communication we discuss some particular features of electron scattering on the nitrogen N2 molecule (also in metastable N2* and ionized N2+ states). A more complete presentation of total and partial cross sections for electron interaction with these species will be given elsewhere [12].
2 Total and elastic cross sections
Studies of electron scattering on N2 brought in the past several surprises, starting from the vibrational structure [13] seen in the total cross section around 2.4 eV collision energy, see Fig. 2. This, 2Пg temporary negative N2− ion became a prototype of so-called shape resonances, i.e., of capturing the incoming electron into an effective scattering potential of the target. In N2 another resonance type, Feshbach resonances, which are related to the excited electronic states of the molecule, are present as sharp structures of a few meV width in the total cross section, see, for example the graphical abstract of measurements at 11.5 eV by Kitajima et al. [14].
Total and elastic cross sections in the region of 2Пg resonance, and in the limit of zero energy. Recent experimental total cross sections by Kitajima et al. [14] agree well with the recommended elastic cross sections of Kawaguchi et al. [16], magenta line, and with recommended (“present”) elastic cross sections [12], squares; for earlier total cross section measurements, see Ref. [19]. We show also the predictions of the modified effective-range theory [20] in two alternatives: assuming the p-wave (blue, thick broken line) and the d-wave (red, broken line). Theoretical vibrational cross sections are by Laporta et al. [23]
In contrast to our previous, similar paper on electron scattering on H2O molecule [15], for which we were able to give only “recommended” cross sections, the state of knowledge on N2 cross sections is quite satisfactory. In H2O, the cross sections (total, elastic, rotational excitation) in the limit of meV energy range are still subject to big (mainly experimental) uncertainties: This is due to the polar character of that molecule. In N2 this is not the case: The cross sections are well determined practically down to the zero energy. The recent, ultra-low energy total cross sections of Kitajima et al. [14] agree with the recommended data of Kawaguchi et al. [16] and with elastic cross sections evaluated by present authors [12] via reviewing available experimental data, see Fig. 2.
The total cross section has been measured by nearly all laboratories working on electron scattering: The 2Пg pronounced vibrational structure is used as the normalization of the energy scale in different experiments. The reference measurement is that by Kennerly [17] from 1980, obtained using a time-of-flight method. The recent experiment performed by Kitajima et al. [14] with 9 meV energy resolutions may serve as a refined normalization. Kitajima et al. determined their energy scale by measuring a very narrow, Feshbach resonance at 14.497 ± 0.002 eV, which was studied earlier both experimentally and theoretically. The maxima of the 2Пg resonant structure reported by Kitajima et al. are, on average, slightly (by − 10 meV) shifted in respect to those by Kennerly (see Table 2 in Ref. [14]). Note however that this difference is still comparable to the declared determination of the energy scale in the experiment of Kitajima et al. (which is ± 5 meV).
A peculiar feature of the 2Пg resonance in N2 is the absence of the dissociative attachment process, in contrast to similar resonances in CO2 or OCS (see [18]); no negative ions were detected at higher impact energies either, again in contrast to H2 or CO [19].
The study of the cross section in the zero-energy limit has more heuristic than practical motivation. The nitrogen molecule, together with Ne, shows a very small but still positive, scattering length of 0.404 a0 [20]. This is in spite of its much higher polarizability (11.5 a03) compared to neon (polarizability 2.575 a03, and scattering length 0.222 a0 [19]).
We will not discuss details of total cross sections at higher energies, as the agreements between different experiment are exemplarily good; see Ref. [12] for details.
3 Vibrational excitation
The nitrogen molecule is symmetric and homo-nuclear, so its permanent dipole moment is null and the direct vibrational excitation should be small. However, N2 shows huge enhancement of the vibrational cross sections via resonances [13, 21, 22]. In the region of the 2Πg resonance the sum of the vibrational excitations amounts to as much as 1/3 of the total cross section, see Fig. 2. Further, higher overtones are excited very efficiently; say, the maximum the v = 4 overtone is 1/4 of that for the v = 0 → 1 excitation, see Fig. 3; overtones up to v = 17 have been observed experimentally [21]. Therefore, the nitrogen molecule can assure efficient cooling of plasmas, and for this reason its use is under consideration for thermonuclear reactors.
Integral cross sections for the vibrational excitation from the v = 0 state of N2, in the region of the 2Πg shape resonance: theoretical data by Laporta et al. [23], in perfect agreement with measurements [21], not shown for clarity. The summed cross section comprises excitations to states up to v = 10 (higher excitations are negligible in this scale)
Cross sections for transitions between all 59 vibrational levels of the ground electronic state were calculated by Laporta et al. [23]: They are in excellent agreement with the available measured cross sections [21, 22]. For this reason, in Fig. 3 we present only theoretical data for selected vibrational excitations from the v = 0 state. Excitations to higher than v = 10 states contribute in insignificant manner to the summed cross section, even if they were detected already in early experiments, see Fig. 19 in the review [19].
4 Electronic excitations
Thresholds for electronic excitations in N2 are pretty high: the lowest, to the triplet A 3Σu+ state having the experimental value of 6.2 eV. This is much higher than, for example, thresholds for NO or NO2, see Ref. [24].
As said in the introduction, the knowledge of the cross sections not only for the electronic excitation but also for the transitions between excited states is fundamental for modeling nitrogen plasmas. Two laboratories performed recently comprehensive studies. JET Propulsion Laboratory from California reported three sets of data: Johnson et al. [25] for the eight low-laying states (A 3Σu+, B 3Πg, W 3Δu, B’ 3Σu−, a 1Πg, a’ 1Σu−, w 1Δu and C 3Πu) between 10 and 100 eV collision energy, Malone et al. [26] for the C 3Πu, E 3Σg+ and a’’ 1Σg+ states, and Malone et al. [27] for high-laying states: b 1Πu, c3 1Πu, o3 1Πu, b’ 1Σu+, c4′ 1Σu+, G 3Πu, F 3Πu between 17.5 and 100 eV collision energy. The Flinders University group [28] re-analyzed elastic cross sections at 15–50 eV collision energy for 10 states (A 3Σu+, B 3Πg, W 3Δu, B’ 3Σu−, a 1Πg, a’ 1Σu−, w 1Δu, C 3Πu, E 3Σg+ and a’’ 1Σg+). The agreement between the two laboratories is fairly good (essentially within combined error bars).
The most comprehensive understanding of electronic excitations comes from the theory: UK R-matrix method calculations yielded cross sections for the excitations from the ground state [29] and transitions from the excited states [30, 31]. Generally, the agreement between different theories and the most recent experiments is good, see Fig. 4. The theories yield the threshold energies that agree with photo-absorption experiments, but are by some 1–2 eV higher than the thresholds observed in electron scattering. One would hypothesize effects of a dynamic polarization of the target molecule by the incoming electron, which lowers the thresholds. Note that in a similar way the experiments on electron-induced ionization give lower thresholds than the semi-empirical approach (Born–Bethe binary encounter model [32]). Further, in Fig. 5 we compare the 2Пg resonance as seen in total cross section with that predicted by the R-matrix suite [30]: The experimental position of the resonance is lower than the calculated one.
Integral cross section for the electronic excitation. Experimental data by Johnson et al. [25]. Theory by Su et al. [30], and Tashiro and Morokuma [50]. Recommended data were given by Itikawa [51] and Kawaguchi et al. [16]. Note that we have arbitrarily shifted the X scale of the theoretical and recommended data by − 1.5 eV, to fit to the experimentally determined excitation threshold
Electron scattering from the ground X 1Σg+ and electronically excited a' 1Σu− and A 3Σu+ states: elastic and selected electronic transitions. Data by Su et al. [30]. Note there is discrepancy between the elastic cross section for the A 3Σu+ state, as in Fig. 7 of Ref. [30] and the supplementary data of that paper: We used the latter sets which correspond to the correct results from the calculation [30]
In Fig. 5 we compare different theories with the JET Propulsion Laboratory measurements for the excitation to the lowest triplet state, A 3Σu+ state. Note, that on that figure we have, arbitrarily, shifted all theories by − 1.5 eV. With this shift, one obtains good agreement between theories and the experiment: The cross section for the excitations to the A 3Σu+ state shows a near-to-threshold resonant enhancement, followed by a broad maximum (and slow “decay,” that would be typical for dipole-allowed transitions). Note that the excitations from the ground (X 1Σg+) state to all (A 3Σu+, B 3Πg, W 3Δu, B’ 3Σu−, a 1Πg, a’ 1Σu−, w 1Δu and C 3Πu) states reported by Johnson et al. [25] show, somewhat surprisingly, the energy dependences typical for the dipole-allowed transitions. The summed cross section for the excitation to these states amounts to as much as 1 × 10–16 cm2 at 20 eV [25].
Electronic transitions (cascading) between different (including metastable) states influence significantly nitrogen plasmas. Some of these states are long-lived: A 3Σu+—2.4 s, a 1Πg—56 μs and a' 1Σu+—20 ms, see Ref. [33]. The metastable a 1Πg state, with the threshold of 8.6 eV, in conditions of electrical discharges may be immediately quenched to the a' 1Σu+ state [34], with almost overlapping vibronic levels. Extensive calculations of electronic transitions from the a 1Πg and A 3Σu+ states to 7 other excited states (plus the de-excitation to the ground state and elastic scattering) were recently given by Su et al. [30]. We show a sample of these results in Fig. 5.
As seen from the figure, the elastic cross section for scattering on the a 1Πg state is between 0 and 10 eV higher that the total cross section for the ground N2 state; the transition (cascading) to the a' 1Σu− state shows a maximum of cross sections at 3 eV (that is a typical energy for medium-temperature plasmas) as high as 10 × 10–16 m2. For more information we refer the reader to the original paper by Su et al. [30], and supplementary data given by them.
5 Dissociation into neutrals
The triple bond in N2 is one of the strongest among molecules: 9.75 eV. In spite of this, the dissociation into neutrals amounts to as much as 10% of the total cross section at 50 eV [35]. However, the uncertainly on this figure is some 20–30%. The early measurement by Winters [36] used the adsorption of dissociate atoms on molybdenum surface and successive bake-out. Cosby [35] used the beam of neutral N2 formed from neutralization of the N2+: This technique may be subject to systematic uncertainties, see Fig. 6.
Integral cross section for dissociation of N2 molecule by electron impact. The recommended data [35] have some 20–30% uncertainty and have been given by Cosby [35] as average between his measurements and those of Winters [36]. The latter measured the sum of the N and N+ signal (both from the ionization processes and from the dissociation into neutrals) while the measurement by Tian and Vidal [52] gave the coincidence of the N+ and N signal. The dissociation into neutrals should amount to the difference between these two determinations: We do not perform the detailed calculation here but a rough comparison shows the congruence between all these [35, 36, 52] sets of data
Already in early measurements [37] three possible dissociation channels were identified:
From the comparison of the release of the translational energy, Cosby [35] concluded that at 50 eV the dominant dissociation pattern is N2 → N(2D) + N(4S); the translational energy spectrum excludes any significant production of the N(4S) + N(4S) dissociation products. Cosby discussed also, on the basis of optical emission experiments, possible electronic excited states that lead to given dissociation channels, but due to fragmentary data this discussion was not conclusive.
A more precise determination of cross sections for the dissociation into neutrals is important also because the higher atomic states are long-lived: The 2DJ state has radiation lifetimes of 17 and 40 h, for the J = 3/2 and J = 5/2 components, respectively [38]. With no doubts, the dissociation into neutrals is the least-studied channel experimentally so far.
6 Ionization and scattering on N2 +
The ionization threshold for the N2 is relatively high 15.58 eV. A peculiar feature of the electron-impact ionization of N2 is a big share of ionization to metastable states of the parent N2+ ion. At 100 eV electron-impact energy the branching ratios for the formation of the ground state ion (X 2Σ+g) and two excited metastable ions (A 2Πu and B 2Σ+u located 1.118 and 3.170 eV above the X state, respectively) are 0.45:0.45:0.1, see Ref. [39].
The total cross section for the ionization of N2 reaches maximum of 2.51 × 10–16 m2 at 100 eV [40], see Fig. 7. We refer to the “total ionization” as the sum of all partial cross sections open at a given energy (and not only the yield of the N2+ ion). The agreement between numerous, different experimental methodologies, both for the total and partial ionizations of N2 is very good. The total ionization is also well reproduced by already cited Born–Bethe binary encounter (BEB) semi-empirical method.
Ionization cross section for the ground state and metastable N2 molecules. Experiments by Straub et al. [40] and Freund et al. [41]. BEB ionization of the metastable by Laricchiuta et al. [42]. The data of Freund et al. for metastables have been (arbitrarily) multiplied by 1.75, to show the agreement (in shape) with BEB
Measurements of the ionization of metastable N2* are few. Freund et al. [41] obtained high (but unknown) concentrations of the metastable A 2Πu nitrogen N2* molecules via resonant charge transfer with triethylamine. They reported the maximum of 1.9 × 10–16 cm2 at 105 eV for the total ionization cross section of N2*. This is less than the maximum for the ground state molecule, and less than the prediction of BEB model [42]. Therefore, in Fig. 7 we show Freund et al. [41] results multiplied by a factor of 1.75. With such an arbitrary “normalization” the agreement between BEB and the experiment would be perfect.
The N2+ ions may undergo in plasmas further reactions: ionization (for example to N22+), dissociation (to N+ and N), dissociative ionization (to two N+ ions) or recombination with electrons (leading to the dissociation of the formed N2 molecule). In specific, the reactions and their thresholds [43] are:
Experiments on these processes are still sporadic. The total ionization has been measured by Peterson et al. at the CRYING storage ring [44]. Results are reported in Fig. 8: They agree very well with the BEB model [45]. The total ionization cross section obtained from “top-table” beam measurements [43] is somewhat lower, see Fig. 8. However, the latter experiment allowed to separate single ionization and/or dissociation channels.
7 Dissociative recombination of N2 + ion
The dissociative recombination of N2+ with very low energy electrons depletes plasmas from electric carriers. Four channels are exothermic, delivering a few eV kinetic energies to atoms formed [33]:
Branching ratios recommended by Dutuit et al. [33] are 0.25, 0.7, 0.05, for 4S, 2D and 2P yields, respectively.
We show the cross sections for the dissociative recombination of the N2+ ion in Fig. 9. In the limit of very low energies (0.001–0.01 eV) it falls down with the collision energy, on average, as 1/√E dependence, and somewhat steeper at higher energies. Experiments on this process are tedious, so we take as the reference theoretical results [46, 47]. The experiment at the CRYING storage ring [44] reached the minimum collision energy of 0.001 eV, with the spread in electron energy of 10 meV: It shows some shoulder of the recombination cross section at 0.05 eV followed by a shallow deep. Top-table measurements with merged beams [48], show similar structures, but their absolute values are by a factor of few folds lower and vary much with different experimental settings, see Fig. 9.
The theory [46, 47, 49] explains it by the strong dependence of absolute values on the initial vibrational state of the N2+ ion: The cross section for the v = 1 state is much lower than for the v = 0 state, see Fig. 8. In the CRYING measurement the population of the two initial states v = 0 and v = 1 was 46% and 27%, respectively. Roughly, the averaging between these two vibrational states would bring the theory to the agreement with the experiment [44] within the uncertainty bar: we do not perform this, as it would smooth-out the specific, resonant-like structures predicted by the theory. We refer the reader to the calculations [47, 49] for detailed cross sections (and reaction rates) for N2+ in different initial vibrational states (and the cross sections for transitions between these states).
8 Conclusions
Nitrogen molecule, apparently chemically inert, yields, via electron scattering, a variety of excited, metastable states—ionized, atomic, molecular. These states influence significantly kinetics of plasmas (plasma torches, microwave, thermonuclear, etc.) and atmospheric processes (on Earth, Solar system satellites, extra-solar planets).
Experiments have obtained a high level of confidence for total, vibrational excitation and ionization cross sections. Theory is highly complementary (in vibrational, electronic excitation, elastic cross sections) and superior in predicting collisional processes in high-temperature plasmas (i.e., for highly excited species). However, significant uncertainties remain for the dissociation into neutrals (and in identifying the branching ratios for products). More experiments are also needed for ionization of metastable molecular states, and on the chemistry involving the molecular ions.
So, in spite of nitrogen molecule being a common gas, electron scattering on it still requires both theories and (new generation) experiments.
Data Availability Statement
This manuscript has no associated data or the data will not be deposited. The datasets generated during the current study will be available from supplementary data linked to Ref. [12].
References
R.A. Armstrong, D.M. Suszcynsky, W.A. Lyons, T.E. Nelson, Geophys. Res. Lett. 27, 653–656 (2000)
A. Tsiaras, I.P. Waldmann, G. Tinetti, J. Tennyson, S.N. Yurchenko, Nature Astron. 3, 1086 (2019)
Z. Li, T. Ozawa, I. Sohn, D.A. Levin, Phys. Fluids 23, 066102 (2011)
S.A. Shcherbanev, M.A. Cherif, S. Starikovskaia, Y. Ikeda, Plasma Sources Sci. Technol. 28, 045009 (2019)
E. Armandillo, A.J. Kearsley, Appl. Phys. Lett. 41, 611 (1982)
T. Fleisch, Y. Kabouzi, M. Moison, J. Castaños-Martínez, H. Nowakowska, Z. Zakrzewski, Plasma Source Sci. Technol. 16, 173 (2006)
V.A. Krasnopolsky, Icarus 236, 83–91 (2014)
U. Dubuet, P. Marietto, M.Y. Perrin, and C. O. Laux, AIAA SCITECH 2022 Forum, San Diego, 3–7 Jan 2022. https://doi.org/10.2514/6.2022-1637. Accessed 25 Mar 2023
T. Sakamoto, H. Matsuura, H. Akatsuka, J. Appl. Phys. 101, 023307 (2007)
G. Colonna, V. Laporta, R. Celiberto, M. Capitelli, J. Tennyson, Plasma Source Sci. Technol. 24, 035004 (2015)
J.M. Ajello, J.S. Evans, V. Veibell, C.P. Malone, G.M. Holsclaw, A.C. Hoskins et al., J. Geophys. Res. Space Phys. 125, e2019JA027546 (2020)
M.-Y. Song, H. Cho, G.P. Karwasz, V. Kokoouline, J. Tennyson, J. Phys. Chem. Ref. Data (2023)
G.J. Schulz, Phys. Rev. 125, 229 (1962)
M. Kitajima, T. Kishino, T. Okumura, N. Kobayashi, A. Sayama et al., Eur. Phys. J. D 71, 139 (2017)
M.-Y. Song, J.-S. Yoon, H. Cho, G.P. Karwasz, V. Kokoouline, Y. Nakamura, J. Tennyson, Eur. Phys. J. D 74, 60 (2020)
S. Kawaguchi, K. Takahashi, K. Satoh, Plasma Sources Sci. Technol. 30, 035010 (2021)
R.E. Kennerly, Phys. Rev. A 21, 1876 (1980)
G.P. Karwasz, R.S. Brusa, A. Zecca, Riv. Nuovo Cimento 24(1), 1–118 (2001)
A. Zecca, G.P. Karwasz, R.S. Brusa, Riv. Nuovo Cimento 19(5), 1–146 (1996)
Z. Idziaszek, G.P. Karwasz, Eur. Phys. J. D 51, 347 (2009)
M. Allan, J. Phys. B: At. Mol. Phys. 18, 4511 (1985)
M. Vićić, G. Poparić, D.D. Belić, J. Phys. B: At. Mol. Opt. Phys. 29, 1273 (1996)
V. Laporta, D. Little, R. Celiberto, J. Tennyson, Plasma Sources Sci. Technol. 23, 065002 (2014)
M.-Y. Song, J.S. Yoon, H. Cho, G.P. Karwasz, V. Kokoouline, Y. Nakamura, J. Tennyson, J. Phys. Chem. Ref. Data 48, 043104 (2019)
P.V. Johnson, C.P. Malone, I. Kanik, K. Tran, M.A. Khakoo, J. Geophys. Res. 110, A11311 (2005)
C. P. Malone, P. Johnson, J. Young, X. Liu, B. Ajdari, M. Khakoo, I. Kanik, J. Phys. B At., Mol. Opt. Phys. 42, 225202 (2009)
C.P. Malone, P.V. Johnson, X. Liu, B. Ajdari, I. Kanik, M.A. Khakoo, Phys. Rev. A 85, 062704 (2012)
L. Campbell, M.J. Brunger, A.M. Nolan, L.J. Kelly, A.B. Wedding, J. Harrison, P.J.O. Teubner, D.C. Cartwright, B. McLaughlin, J. Phys. B: At. Mol. Opt. Phys. 34, 1185 (2001)
C.J. Gillan, J. Tennyson, B.M. McLaughlin, P.G. Burke, J. Phys. B: At. Mol. Opt. Phys. 29, 1531–1547 (1996)
H. Su, X. Cheng, H. Zhang, J. Tennyson, J. Phys. B: At. Mol. Opt. Phys. 54, 115203 (2021)
H. Su, X. Cheng, B. Cooper, J. Tennyson, H. Zhang, Phys. Rev. A 105, 062824 (2022)
D. Gupta, H. Choi, M.-Y. Song, G.P. Karwasz, J.-S. Yoon, Eur. Phys. J. D 71(4), 70769–70776 (2017)
O. Dutuit, N. Carrasco, R. Thissen, V. Vuitton, C. Alcaraz, P. Pernot et al., Astrophys. J. Suppl. 204, 20 (2013)
W.J. Martinelli, W.J. Kessler, B.D. Green, W.A.M. Blumberg, J. Chem. Phys. 91, 701 (1989)
P.C. Cosby, J. Chem. Phys. 98, 9544 (1993)
H.F. Winters, J. Chem. Phys. 44, 1472 (1966)
D.C. Frost, C.A. McDowell, Proc. R. Soc. Lond. A 236, 278 (1956)
U. Bley, M. Koch, F. Temps, P.B. Davies, I.H. Davis, J. Chem. Phys. 90, 628 (1989)
J.P. Doering, L. Goembel, J. Geophys. Res. Space Phys. 96(A9), 16025 (1991)
H.C. Straub, P. Renault, B.G. Lindsay, K.A. Smith, R.F. Stebbings, Phys. Rev. A 54, 2146 (1996)
R.S. Freund, R.C. Wetzel, R. Shul, Phys. Rev. A 41, 3575 (1990)
A. Laricchiuta, R. Celiberto, G. Colonna, Atoms 10(2), 10010002 (2022)
E.M. Bahati, J.J. Jureta, D.S. Belić, H. Cherkani-Hassani, M.O. Abdellahi, P. Defrance, J. Phys. B: At. Mol. Opt. Phys. 39, 2963 (2001)
J.R. Peterson, A. Le Padellec, H. Danared, G.H. Dunn, M. Larsson et al., J. Chem. Phys. 108, 1978–1988 (1998)
Y.-K. Kim, K.K. Irikura, M.A. Ali, J. Res. NIST 105, 285 (2000)
S.L. Guberman, J. Chem. Phys. 139, 124318 (2013)
D.A. Little, K. Chakrabarti, J.Z. Mezei, I.F. Schneider, J. Tennyson, Phys. Rev. A 90, 052705 (2014)
C. Noren, F.B. Yousif, B.A. Mitchell, J. Chem. Soc. Faraday Trans. 85, 1697 (1989)
A. Abdoulanziz, C. Argentin, V. Laporta, K. Chakrabarti, A. Bultel, J. Tennyson, I.F. Schneider, J.Z. Mezei, J. Appl. Phys. 129, 053303 (2021)
M. Tashiro, K. Morokuma, Phys. Rev. A 75, 012720 (2007)
Y. Itikawa, J. Phys. Chem. Ref. Data 35, 31 (2006)
C. Tian, C.R. Vidal, J. Phys. B: At. Mol. Opt. Phys. 31, 5369 (1998)
Acknowledgments
One of us (VK) acknowledges the U.S. National Science Foundation, Grants Nos. 2110279 and 2102188.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the paper.
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, MY., Cho, H., Karwasz, G.P. et al. Electron scattering on molecular nitrogen: common gas, uncommon cross sections. Eur. Phys. J. D 77, 105 (2023). https://doi.org/10.1140/epjd/s10053-023-00687-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjd/s10053-023-00687-5