Abstract
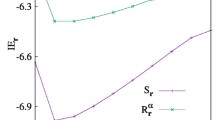
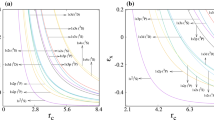
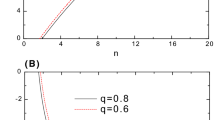
The present work studies aspects of the electronic correlation in confined \(\hbox {H}^{-}\), He and \(\hbox {Li}^+\) atoms in their ground states using the informational entropies. In this way, different variational wavefunctions are employed in order of better take account of Coulomb correlation. The obtained values for the \(S_r\), \(S_p\) and \(S_t\) entropies are sensitive in relation to Coulomb correlation effects. In the strong confinement regime, the effects of the Coulomb correlation are negligible and the employment of the models of independent particle and two non-interacting electrons confined by a impenetrable spherical cage gains importance in this regime. Lastly, energy values are obtained in good agreement with the results available in the literature.
Graphic abstract








Similar content being viewed by others
Data Availability Statement
This manuscript has data included as electronic supplementary material.
References
P. Fulde, Electron Correlation in Molecules and Solids (Springer, Berlin, 1995). https://doi.org/10.1007/978-3-642-57809-0
R.P. Sagar, H.G. Laguna, N.L. Guevara, Chem. Phys. Lett. 514, 352 (2011). https://doi.org/10.1016/j.cplett.2011.08.032
D.P. Tew, W. Klopper, T. Helgaker, J. Comp. Chem. 28, 1307 (2007). https://doi.org/10.1002/jcc.20581
L. Rincon, F.J. Torres, M. Becerra, S. Liu, A. Fritsch, R. Almeida, Mol. Phys. 117(5), 610 (2019). https://doi.org/10.1080/00268976.2018.1530462
P.O. Löwdin, Adv. Chem. Phys. 2, 207 (1958). https://doi.org/10.1002/9780470143483.ch8
A.L. Baskerville, A.W. King, H. Cox, R. Soc. opens ci. 6, 181357 (2019). https://doi.org/10.1098/rsos.181357
A. Mohajeri, M. Alipour, J. Mol. Struct.: THEOCHEM 907, 115 (2009). https://doi.org/10.1016/j.theochem.2009.04.036
S.P. McCarthy, A.J. Thakkar, Chem. Phys. Lett. 494, 312 (2010). https://doi.org/10.1016/j.cplett.2010.05.095
O. Rioul, Théorie de l’information et du codage (Lavoisier, Paris, 2007)
C. Shannon, Bell Syst. Tech. J. 27, 379 (1948). https://doi.org/10.1002/j.1538-7305.1948.tb01338.x
Z.X. Wang, L.Y. He, D.D. Li, Energy Policy 132, 429 (2019). https://doi.org/10.1016/j.enpol.2019.06.002
Z. Liu, P. Shang, Phys. A (Amsterdam, Neth.) 505, 1170 (2018). https://doi.org/10.1016/j.physa.2018.04.041
O. Olendski, Ann. Phys. (Berlin) 530, 1700324 (2018). https://doi.org/10.1002/andp.201700324
W.S. Nascimento, M.M. de Almeida, F.V. Prudente, Eur. J. Phys. 41(2), 025405 (2020). https://doi.org/10.1088/1361-6404/ab5f7d
A. Ghosal, N. Mukherjee, A.K. Roy, Ann. Phys. (Berlin) 528(11–12), 796 (2016). https://doi.org/10.1002/andp.201600121
H.G. Laguna, R.P. Sagar, Ann. Phys. (Berlin) 526(11–12), 555 (2014). https://doi.org/10.1002/andp.201400156
G.H. Sun, S.H. Dong, N. Saad, Ann. Phys. (Berlin) 525(12), 934 (2013). https://doi.org/10.1002/andp.201300089
C.R. Estañón, N. Aquino, D.D. Puertas-Centeno, J.S. Dehesa, Int. J. Quantum Chem. 120(11), e26192 (2020). https://doi.org/10.1002/qua.26192
N. Mukherjee, A.K. Roy, Eur. Phys. J. D 72, 118 (2018). https://doi.org/10.1140/epjd/e2018-90104-1
M.A. Martínez-Sánchez, R. Vargas, J. Garza, Quantum Rep. 1, 208 (2019). https://doi.org/10.3390/quantum1020018
W.S. Nascimento, F.V. Prudente, Chem. Phys. Lett. 691, 401 (2018). https://doi.org/10.1016/j.cplett.2017.11.048
S.J.C. Salazar, H.G. Laguna, V. Prasad, R.P. Sagar, Int. J. Quantum Chem. 120(11), e26188 (2020). https://doi.org/10.1002/qua.26188
J.S. Dehesa, E.D. Belega, I.V. Toranzo, A.I. Aptekarev, Int. J. Quantum Chem. 119(18), e25977 (2019). https://doi.org/10.1002/qua.25977
J.H. Ou, Y.K. Ho, Int. J. Quantum Chem. 119(14), e25928 (2019). https://doi.org/10.1002/qua.25928
I. Nasser, M. Zeama, A.A.-Hady, Mol. Phys. 118(3), 1612105 (2020). https://doi.org/10.1080/00268976.2019.1612105
I. Nasser, M. Zeama, A. A.-Hady, Results Phys. 7, 3892 (2017). https://doi.org/10.1016/j.rinp.2017.10.013
K.D. Sen, J. Chem. Phys. 123, 074110 (2005). https://doi.org/10.1063/1.2008212
S. Majumdar, A. Roy, Quantum Rep. 2, 189 (2020). https://doi.org/10.3390/quantum2010012
C.H. Lin, Y.K. Ho, Chem. Phys. Lett. 689, 116 (2017). https://doi.org/10.1016/j.cplett.2017.10.007
J.H.O. Lin, Y.K. Ho, Atoms 5, 15 (2017). https://doi.org/10.3390/atoms5020015
I. Nasser, M. Zeama, A. Abdel-Hady, Phys. Scr. 95(9), 095401 (2020). https://doi.org/10.1088/1402-4896/abaa09
C. Martínez-Flores, Phys. Lett. A 386, 126988 (2021). https://doi.org/10.1016/j.physleta.2020.126988
C. Martínez-Flores, M. Martínez-Sánchez, R. Vargas, J. Garza, Eur. Phys. J. D 75, 100 (2021). https://doi.org/10.1140/epjd/s10053-021-00110-x
A. Michels, J. de Boer, A. Bijl, Physica (Amsterdam) 4, 981 (1937). https://doi.org/10.1016/S0031-8914(37)80196-2
V. Fock, Z. Phys. 47, 446 (1928). https://doi.org/10.1007/BF01390750
C.G. Darwin, Math. Proc. Cambridge Philos. Soc. 27, 86 (1931). https://doi.org/10.1017/S0305004100009373
R. Dutt, A. Mukherjee, Y.P. Varshni, Phys. Lett. A 280, 318 (2001). https://doi.org/10.1016/S0375-9601(01)00067-6
J.F. da Silva, F.R. Silva, E. Drigo Filho, Int. J. Quantum Chem. 116, 497 (2016). https://doi.org/10.1002/qua.25084
A. Flores-Riveros, A. Rodríguez-Contreras, Phys. Lett. A 372(40), 6175 (2008). https://doi.org/10.1016/j.physleta.2008.08.027
M.F. Hasoglu, H.L. Zhou, S.T. Manson, Phys. Rev. A 93, 022512 (2016). https://doi.org/10.1103/PhysRevA.93.022512
S.B. Doma, F.N. El-Gammal, J. Theor. Appl. Phys. 6, 28 (2012). https://doi.org/10.1186/2251-7235-6-28
J.P. Connerade, Eur. Phys. J. D 74, 211 (2020). https://doi.org/10.1140/epjd/e2020-10414-y
S. Saha, J. Jose, Int. J. Quantum Chem. 120(22), e26374 (2020). https://doi.org/10.1002/qua.26374
M. Rodriguez-Bautista, R. Vargas, N. Aquino, J. Garza, Int. J. Quantum Chem. 118(13), e25571 (2018). https://doi.org/10.1002/qua.25571
S. LumbTalwar, S. Lumb, V. Prasad, Eur. Phys. J. D 75, 59 (2021). https://doi.org/10.1140/epjd/s10053-021-00064-0
A.M. Maniero, C.R. de Carvalho, F.V. Prudente, Ginette Jalbert, J. Phys. B: At., Mol. Opt. Phys 53(18), 185001 (2020). https://doi.org/10.1088/1361-6455/ab9f0f
H. de Oliveira Batael, E. Drigo Filho, J. Chahine, Eur. Phys. J. D 75, 52 (2021). https://doi.org/10.1140/epjd/s10053-021-00057-z
F.V. Prudente, M.N. Guimarães, in Electronic Structure of Quantum Confined Atoms and Molecules, ed. by K.D. Sen (Springer, Cham, 2014), chap. 5, pp. 101–143. https://doi.org/10.1007/978-3-319-09982-8_5
R. Hernández-Esparza, B. Landeros-Rivera, R. Vargas, J. Garza, Ann. Phys. (Berlin) 531(7), 1800476 (2019). https://doi.org/10.1002/andp.201800476
L.F. Pasteka, T. Helgaker, T. Saue, D. Sundholm, H.J. Werner, M. Hasanbulli, J. Major, P. Schwerdtfeger, Mol. Phys. 118(19–20), e1730989 (2020). https://doi.org/10.1080/00268976.2020.1730989
W.S. Nascimento, F.V. Prudente, Quim. Nova 39, 757 (2016). https://doi.org/10.5935/0100-4042.20160045
S. Saha, J. Jose, Phys. Rev. A 102, 052824 (2020). https://doi.org/10.1103/PhysRevA.102.052824
N.L. Guevara, R.P. Sagar, R.O. Esquivel, J. Chem. Phys. 122, 084101 (2005). https://doi.org/10.1063/1.1848092
L.D. Site, Int. J. Quantum Chem. 115(19), 1396 (2014). https://doi.org/10.1002/qua.24823
H.T. Peng, Y.K. Ho, Entropy 17, 1882 (2015). https://doi.org/10.3390/e17041882
A. Mohajeri, M. Alipour, Chem. Phys. 360, 132 (2009). https://doi.org/10.1016/j.chemphys.2009.04.016
R.P. Sagar, N.L. Guevara, J. Chem. Phys. 124, 134101 (2006). https://doi.org/10.1063/1.2180777
R.O. Esquivelt, A.N. Tripathi, R.P. Sagar, V.H.J. Smith, J. Phys. B: At. Mol. Opt. Phys. 25, 2925 (1992). https://doi.org/10.1088/0953-4075/25/13/003
A.N. Tripathi, R.P. Sagar, R.O. Esquivel, V.H.J. Smith, Phys. Rev. A 45, 4385 (1992). https://doi.org/10.1103/PhysRevA.45.4385
R.P. Sagar, M. Hô, J. Mex. Chem. Soc. 52(1), 60 (2008)
I. Bialynicki-Birula, J. Mycielski, Commun. Math. Phys. 44, 129 (1975). https://doi.org/10.1007/BF01608825
H.H. Corzo, H.G. Laguna, R.P. Sagar, J. Math. Chem. 50, 233 (2012). https://doi.org/10.1007/s10910-011-9908-2
E.A. Hylleraas, in Advances in Quantum Chemistry, vol. 1, ed. by P.O. Löwdin (Academic Press, 1964), pp. 1–33. https://doi.org/10.1016/S0065-3276(08)60373-1
F.S. Carvalho, J.P. Braga, Quim. Nova 40(10), 1259 (2017). https://doi.org/10.21577/0100-4042.20170125
C.A. Ten Seldam, R. De Groot, Physica 18(11), 891 (1952). https://doi.org/10.1016/S0031-8914(52)80223-X
X.Y. Pan, V. Sahni, L. Massa, arXiv:physics/0310128 (2003)
B.M. Gimarc, J. Chem. Phys. 47(12), 5110 (1967). https://doi.org/10.1063/1.1701767
S. Chandrasekhar, Astrophys. J. 100, 176 (1944). https://doi.org/10.1086/144654
R.N. Hill, Phys. Rev. Lett. 38(12), 643 (1977). https://doi.org/10.1103/PhysRevLett.38.643
H.A. Bethe, E.E. Salpeter, Quantum Mechanics of One- and Two- electron Atoms (Springer-Verlag, Berlin, 1977)
C. Le Sech, A. Banerjee, J. Phys. B: At. Mol. Opt. Phys. 44(10), 105003 (2011). https://doi.org/10.1088/0953-4075/44/10/105003
C.N. Isonguyo, K.J. Oyewumi, O.S. Oyun, Int. J. Quantum Chem. 118(15), e25620 (2018). https://doi.org/10.1002/qua.25620
N. Aquino, A. Flores-Riveros, J. Rivas-Silva, Phys. Lett. A 377(34), 2062 (2013). https://doi.org/10.1016/j.physleta.2013.05.048
F.G.F.A. de Saavedra, E. Buendía, Z Phys D - Atoms. Molecules and Clusters 38, 25 (1996). https://doi.org/10.1007/s004600050058
C.H. Lin, Y.K. Ho, Chem. Phys. Lett. 633, 261 (2015). https://doi.org/10.1016/j.cplett.2015.05.029
R.G. Parr, Y. Weitao, Density-functional theory of atoms and molecules. International series of monographs on chemistry 16 (Oxford University Press, 1994)
Á. Nagy, Phys. Rep. 298(1), 1 (1998). https://doi.org/10.1016/S0370-1573(97)00083-5
N.L. Guevara, R.P. Sagar, R.O. Esquivel, J. Chem. Phys. 119(14), 7030 (2003). https://doi.org/10.1063/1.1605932
H. Montgomery, N. Aquino, A. Flores-Riveros, Phys. Lett. A 374(19), 2044 (2010). https://doi.org/10.1016/j.physleta.2010.02.074
N. Aquino, A. Flores-Riveros, J. Rivas-Silva, Phys. Lett. A 307(5), 326 (2003). https://doi.org/10.1016/S0375-9601(02)01767-X
A. Flores-Riveros, N. Aquino, H. Montgomery, Phys. Lett. A 374(10), 1246 (2010). https://doi.org/10.1016/j.physleta.2009.12.062
T.N. Barbosa, M.M. Almeida, F.V. Prudente, J. Phys. B: At., Mol. Opt. Phys. 48(5), 055002 (2015). https://doi.org/10.1088/0953-4075/48/5/055002
F.V. Prudente, L.S. Costa, J.D.M. Vianna, J. Chem. Phys. 123(22), 224701 (2005). https://doi.org/10.1063/1.2131068
Acknowledgements
This work has been supported by the Brazilian agencies CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through grants to the authors. The authors thank the referees for careful reading of the manuscript and for helpful comments and suggestions.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the paper.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix A:
Appendix A:
The \(2e^{-}\) system consists of two non-interacting electrons confined in an impenetrable spherical cage. The non-relativistic Hamiltonian in atomic units for one electron confined in an impenetrable spherical cage, \(e^{-}\), is
The confining potential is defined as
being \(r_c \) the confinement radius. The ground state wavefunction is
where A is a normalization constant. In this background, for the \(2e^{-}\) system the expectation values of the energy, beyond the entropic quantities, are the double of the values obtained for the \(e^{-}\) system. The \(S_r\), \(S_p\), \(S_t\) and \(\langle E \rangle \) values as a function of \(r_c\) for the \(2e^{-}\) system in ground state are available in Table S5 of the Supplementary Material.
Rights and permissions
About this article
Cite this article
Nascimento, W.S., de Almeida, M.M. & Prudente, F.V. Coulomb correlation and information entropies in confined helium-like atoms. Eur. Phys. J. D 75, 171 (2021). https://doi.org/10.1140/epjd/s10053-021-00177-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjd/s10053-021-00177-6