Abstract
Solidification of the melted two-dimensional square lattice structure forming system is studied using molecular dynamics simulations. The initial model of 6400 atoms interacting via the square potential proposed by Rechstmann et al. (Phys. Rev. E, 73:011406, 2006) is cooled down from the melt at two different cooling rates. The research reveals the differences in the evolution of structure and thermodynamics of the system upon cooling from the melt. At the cooling rate of \({10}^{-7}\) per MD step, the phase transition temperature is found to be \({T}_{C}=0.50\), while it is \({T}_{g}=0.43\) at the cooling rate of \({10}^{-5}\) per MD step. Atomic mechanism of solidification of the system is analyzed via studying of the occurrence and growth of solid-like atoms upon cooling from the melt. Three characteristic temperatures of solidification are proposed. There is an evidence of the first-order phase transition behavior of the crystallization of the 2D melt.
Graphical abstract

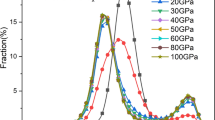
T/TX dependence of the fraction of solid-like atoms (NS is the number of solid-like atoms, N is the total number of atoms in the system, T is temperature, TX is equal to Tg = 0.43 or TC = 0.50 depending on the cooling rate)












Similar content being viewed by others
Data availability statement
The data supporting to this study’s findings cannot be openly available due to the rules of the funding foundation and are available from the corresponding author upon request.
References
M. Rechtsman, F. Stillinger, S. Torquato, Phys. Rev. E 73, 011406 (2006)
G. Algara-Siller, O. Lehtinen, F.C. Wang, R.R. Nair, U. Kaiser, H.A. Wu, I.V. Grigorieva, A.K. Geim, Nature 519, 443 (2015)
J. Chen, G. Schusteritsch, C.J. Pickard, C.G. Salzmann, A. Michaelides, Phys. Rev. Lett. 116, 025501 (2016)
H. Ohnishi, Y. Kondo, T. Kunio, Nature 395, 780 (1998)
M.J. Lagos, F. Sato, D.S. Galvão, D. Ugarte, Phys. Rev. Lett. 106, 055501 (2011)
J. Zhao, Q. Deng, A. Bachmatiuk, G. Sandeep, A. Popov, J. Eckert, M.H. Rümmeli, Science 343, 1228 (2014)
Y. Shao, R. Pang, X. Shi, J. Phys. Chem. C. 119, 22954 (2015)
V. van Hoang, N.T. Hieu, J. Phys. Chem. C 120, 18340 (2016)
P. Wang, H. Wang, W. Yang, RSC Adv. 4, 17008 (2014)
M.R. Thomsen, S.J. Brun, T.G. Pedersen, Phys. Rev. B 91, 125439 (2015)
H. Zhang, Y.M. Dai, L.M. Liu, Comp. Mater. Sci. 101, 255 (2015)
K. Takahashi, T. Hussain, L. Takahashi, J.D. Baran, Cryst. Growth Des. 16(3), 1746 (2016)
A. Quandt, M.P. Teter, Phys. Rev. B 59(13), 8586 (1999)
A. Jain, J.R. Errington, T.M. Truskett, Phys. Rev. X. 4, 031049 (2014)
T. Kawasaki, H. Tanaka, PNAS 107(32), 14036 (2010)
P. Tan, N. Xu, L. Xu, Nat. Phys. 10, 73 (2013)
E. Sanz, C. Valeriani, Nat. Mater. 14, 15 (2015)
W. Qi, Y. Peng, Y. Han, R.K. Bowles, M. Dijkstra, Phys. Rev. Lett. 115, 185701 (2015)
T.A. Weber, F.H. Stillinger, Phys. Rev. E 48, 4351 (1993)
M. Engel, H.-R. Trebin, Phys. Rev. Lett. 98, 225505 (2007)
W. Humphrey, A. Dalke, K. Schulten, J. Mol. Graph. 14, 33 (1996)
D.E. Dudalov, E.N. Tsiok, Y.D. Fomin, V.N. Ryzhov, J. Chem. Phys. 141, 18C522 (2014)
V. van Hoang, V. Teboul, T. Odagaki, J. Phys. Chem. B 119, 15752 (2015)
J.J. Gilvarry, Phys. Rev. 102(2), 308 (1956)
F.H. Stillinger, T.A. Weber, Phys. Rev. B 22(8), 3790 (1980)
R. Agrawal, D.A. Kofke, Mol. Phys. 85, 43 (1995)
P.G. Bolhuis, D.A. Kofke, Phys. Rev. E 54(1), 634 (1996)
D.A. Kofke, P.G. Bolhuis, Phys. Rev. E 59(1), 618 (1999)
C. Chakravarty, J. Chem. Phys. 116, 8938 (2002)
Z. Wang, A.M. Alsayed, A.G. Yodh, Y. Han, J. Chem. Phys. 132, 154501 (2010)
P. Dillmann, G. Maret, P. Keim, J. Phys. Condens. Mat. 20, 404216 (2008)
R. Ravinder, R. Kumar, M. Agarwal, N.M.A. Krishnan, Sci. Rep. 9, 4517 (2019)
V.V. Hoang, T. Odagaki, J. Phys. Chem. B 115, 6946 (2011)
S. Tang, M. Wu, S. Bai, D. Luo, J. Zhang, D. Wan, X. Li, J. Mater. Chem. C 10, 16116 (2022)
S. Ono, Sci. Reports 10, 11810 (2020)
V.V. Hoang, T.N. Thanh-Thuy, N.H. Giang, T.Q. Dong, Comp. Mater. Sci. 181, 109730 (2020)
K. Binder, S. Sengupta, P. Nielaba, J. Phys.: Condens. Matter 14, 2323 (2002)
K.J. Strandburg, Rev. Mod. Phys. 60, 161 (1988)
Acknowledgements
This research is funded by Vietnam National University HoChiMinh City (VNU-HCM) under grant number VL2020-20-01.
Author information
Authors and Affiliations
Contributions
This article is written by NTN (PhD student) based on the idea and supervision of Prof. VVH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nga, N.T., Van Hoang, V. Solidification of 2D simple monatomic system: molecular dynamics simulations. Eur. Phys. J. B 96, 82 (2023). https://doi.org/10.1140/epjb/s10051-023-00554-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjb/s10051-023-00554-7