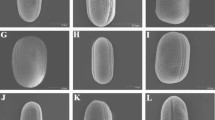
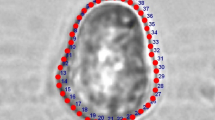
Abstract
By the example of morphological features of pollen of eight species of Nierembergia and two species of Bouchetia (Solanaceae family), the properties of individual variability are described. Most of the studied characters (structure of apertures, ultrastructure of the sporoderm, surface sculpture, and dimensions) do not have significant differences at the individual and intraspecific levels; taxonomically significant variability of morphological features of pollen is manifested at the level of the genus and suprageneric groups. The genera differ significantly in the sculpture of the pollen grain surface—striate in Nierembergia and tuberculate in Bouchetia. Pollen contained in one bud, anther, or tetrad (fully completed gametophytic generation, where there is no death and all without exception descendants of one ancestor are alive), is considered as an extreme model (maximum completeness with minimum complexity) to study the properties of natural morphological variability and the causes of its occurrence. It was found that pollen characters (sculpture, number and location of apertures) have the same pattern of variability (continuous and transitively ordered series), which is embodied at different taxonomic levels in different characters. The natural variability of morphological features of pollen is ordered not into a genealogical clade, but into a cline—continuous, geometrically ordered and transitive series (taxon-nonspecific and rank-independent). In this system of parallelisms, homologous series are inseparable from nonhomologous ones, and typical forms are inseparable from deviations. The origin of typical and deviant forms cannot be explained separately (typical—genealogically, and deviant—as parallelism, convergence, chance or regularity). The individual variability of pollen forms is geometrically ordered and is not the result of random disturbances, failures of the hereditary program, or pathology. The typical form turns out to be a harmonious part of a geometrically ordered series of pure forms, free from functional and historical connotations. The similarity of pollen forms in these series is determined by their geometry and does not depend on affinity, homology, or functionality. The natural system of pollen forms is due not to the structure of supposed affinity of supposed taxa, but the structure of the observed parallelisms of the variability of individual living bodies. Evolutionary novelty (the current state, the observed variability) arises initially ordered in a preestablished form.




Similar content being viewed by others
REFERENCES
Arkhangelskii, D.B., Morphological types of pollen grains of modern flowering plants, Bot. Zh., 1982, vol. 67, no. 7, pp. 890–897.
Berg, L.S., Nomogenez ili evolyutsiya na osnove zakonomernostei (Nomogenesis or Evolution Based on Laws), Petrograd: Gos. Izd., 1922.
Blackmore, S. and Crane, P.R., The evolution of apertures in the spores and pollen grains of embryophytes, in Reproductive Biology, Owens, S.J. and Rudall, P.J., Eds., Kew: Royal Botanic Gardens, 1998, pp. 159–218.
Bunge, M., Prichinnost’. Mesto printsipa prichinnosti v sovremennoi nauke (Causality. The Place of the Causality Principle in Modern Science), Moscow: URSS, 2010.
Chaikovskii, Yu.V., Avtopoez (Autopoiesis), Moscow: KMK, 2018.
Cocucci, A.A., Nierembergia, in Flora Argentina, vol. 13: Dicotyledoneae, Solanaceae, Buenos Aires: Instituto de Botánica Darwinion, 2013, pp. 149–150.
Danilevskii, N.Ya., Darvinizm. Kriticheskoe issledovanie (Darwinism. A Critical Examination), Moscow: FIV, 2015.
Erdtman, G., Pollen Morphology and Taxonomy: Angiosperms, Stockholm: Almquist and Wiksell, 1952.
Finot, V.L., Marticorena, C., and Marticorena, A., Pollen grain morphology of Nolana L. (Solanaceae: Nolanoideae: Nolaneae) and related genera of southern South American Solanaceae, Grana, 2018, vol. 57, no. 6, pp. 415–455. https://doi.org/10.1080/00173134.2018.1458897
Gavrilova, O., Britski, D., Grigorieva, V., Tarasevich, V., Pozhidaev, A., and Leunova, V., Pollen morphology of the genus Euonymus (Celastraceae), Turczaninowia, 2018, vol. 21, no. 4, pp. 188–206. https://doi.org/10.14258/turczaninowia.21.4.20
Goethe, J.W., Nauchnye sochineniya (Scientific Works), vol. 1: Obrazovanie i preobrazovanie organicheskikh sushchestv (morphologiya) (Formation and Transformation of Organic Beings (Morphology)), Moscow: KMK, 2014.
Grigoryeva, V.V., Britskii, D.A., and Korobkov, A.A., Pollen morphology of Artemisia species (Asteraceae) of the Russian Far East, Bot. Zh., 2018, vol. 103, no. 10, pp. 1255–1264. https://doi.org/10.7868/S0006813618100046
Grigoryeva, V.V., Pozhidaev, A.E., Semenov, A.N., and Britskii, D.A., Morphological variability of pollen of Nicotiana (Solanaceae), Bot. Zh., 2019, vol. 104, no. 6, pp. 900–917. https://doi.org/10.1134/S0006813619060061
Hayrapetyan, A.M., Features of the exine ornamentation of pollen grains in the family Solanaceae Juss. I. The simple types of ornamentation, Nat. Sci., 2008, vol. 2, no. 11, pp. 46–50.
Hofman, C.C. and Zetter, R., Upper Cretaceous pollen flora from the Vilui basin, Siberia: Circumpolar and endemic Aquilapollenites, Manicorpus, and Azonia species, Grana, 2007, vol. 47, no. 4, pp. 227–249. https://doi.org/10.1080/00173130701763142
Hunziker, A.T., Genera Solanacearum: The Genera of Solanaceae Illustrated, Arranged according to a New System, Liechtenstein: Ruggell, 2001.
Huynh, K., Le pollen et la systematique du genre Sideritis L. (Labiatae), Bull. Mus. Natl. Hist. Nat., 1972, vol. 3, no. 45, pp. 1–28.
In Memoriam, S.V. Meyen: paleobotanik, evolyutsionist, myslitel’ (S.V. Meyen: Paleobotanist, Evolutionist, Thinker), Moscow: GEOS, 2007.
Kremp, G.O.W., Morphologic Encyclopedia of Palynology, Tucson: The University of Arizeona Press, 1965.
Krenke, N.P., Phenogenetic variability, in Sbornik rabot otdeleniya fitomorfogeneza (Works of the Department of Phytomorphogenesis), Moscow: Biol. Inst. im. Timiryazeva, 1933–1935, vol. 1, pp. 11–415.
Kupriyanova, L.A. and Aleshina, L.A., Palinologicheskaya terminologiya pokrytosemennykh rastenii (Palynological Terminology of Angiosperms), Leningrad: Nauka, 1967.
Kupriyanova, L.A., and Aleshina, L.A., Pyl’tsa i spory rastenii flory evropeiskoi chasti SSSR (Plant Pollen and Spores of the Flora of the European Part of the USSR), Leningrad: Nauka, 1972, vol. 1, pp. 48–51.
Lyubarskii, G.Yu., Proiskhozhdenie ierarkhii: Istoriya taksonomicheskogo ranga (The Origin of Hierarchy: The History of Taxonomic Rank), Moscow: KMK, 2018.
Lyubishchev, A.A., Problemy formy, sistematiki i evolyutsii organizmov (Problems of Form, Systematics, and Evolution of Organisms), Moscow: Nauka, 1982.
Manukyan, L.K., Palynology of the genus Sideritis L., in Palinologiya (Palynology), Yerevan: Akad. Nauk Armyanskoi SSR, 1975, pp. 40–44.
Meyen, S.V., Plant morphology in its nomothetical aspects, Bot. Rev., 1973, vol. 39, no. 3, pp. 205–260.
Meyen, S.V., Fundamental aspects of the typology of organisms, Zh. Obshch. Biol., 1978, vol. 39, no. 4, pp. 495–508.
Oskol’skii, A.A., On the phenomenology of biological similarity, in Gomologii v botanike: opyt i refleksiya (Homologies in Botany: Experience and Reflection), Oskol’skii, A.A., Sokolov, D.D., and Timonin, A.K., Eds., St. Petersburg: Sankt-Peterb. Soyuz Uchenykh, 2001, pp. 115–133.
Pavlinov, I.Ya., Biologicheskaya sistematika: v poiskakh estestvennoi sistemy (Biological Systematics: In Search of a Natural System), Moscow: KMK, 2019.
Peyrot, D., Barrön, E., Comas-Rengifo, M.J., Touand, E., and Tafforeau, P., A confocal laser scanning and conventional wide field light microscopy study of Classopollis from the Toarcian-Aalenian of the Fuentelsaz section (Spain), Grana, 2007, vol. 46, no. 4, pp. 217–226.
Pire, S.M. and Dematteis, M., Pollen aperture heteromorphism in Centaurium pulchellum (Gentianaceae), Grana, 2007, vol. 46, no. 1, pp. 1–12.
Pozdnyakov, A.A., Filosofskoe obosnovanie klassicheskoi biologii: Mekhanitsizm v evolyutsionistike i sistematike (Philosophical Justification of Classical Biology: Mechanism in Evolutionism and Systematics), Moscow: LENAND, 2015.
Pozhidaev, A.E., Structure of the exine of pollen grains of representatives of the family Lamiaceae, Bot. Zh., 1989, vol. 74, no. 10, pp. 1410–1422.
Pozhidaev, A.E., Polymorphism of pollen in the genus Acer (Aceraceae). Isomorphism of deviant forms of Angiosperm pollen, Grana, 1993, vol. 32, no. 1, pp. 79–85. https://doi.org/10.1080/00173139509429028
Pozhidaev, A.E., Pollen morphology of the genus Aesculus (Hippocastanaceae). Patterns in the variety of morphological characteristics, Grana, 1995, vol. 34, no. 1, pp. 10–20. https://doi.org/10.1080/00173139509429028
Pozhidaev, A.E., Hypothetical way of pollen aperture patterning. 1. Formation of 3-colpate patterns and endoaperture geometry, Rev. Palaeobot. Palynol., 1998, vol. 104, no. 1, pp. 67–83.
Pozhidaev, A.E., Hypothetical way of pollen aperture patterning. 2. Formation of polycolpate patterns and pseudoaperture geometry, Rev. Palaeobot. Palynol., 2000a, vol. 109, nos. 3–4, pp. 235–254. https://doi.org/10.1016/s0034-6667(99)00057-3
Pozhidaev, A.E., Pollen variety and aperture patterning, in Pollen and Spores: Morphology and Biology, Harley, M.M., Morton, C.M., and Blackmore, S., Eds., Kew: Royal Botanic Gardens, 2000b, pp. 205–225.
Pozhidaev, A.E., Hypothetical way of pollen aperture patterning. 3. A family-based study of Krameriaceae, Rev. Palaeobot. Palynol., 2002, vol. 127, nos. 1–2, pp. 1–23. https://doi.org/10.1016/S0034-6667(02)00251-8
Pozhidaev, A.E., Pattern of morphological variety of angeospermous pollen aperture distribution and natural ordering of biological variety, or what is a variety (problems of description and interpretation), in Tr. ZIN RAN (Proc. Zool. Inst. Russ. Acad. Sci.), Moscow: KMK, 2009, suppl. 1, pp. 151–182.
Pozhidaev, A.E., Refrain structure of biological variety and the theory of phylogeny, in Paleobotanicheskii vremennik (Paleobotanical Chronicle), Moscow: GEOS, 2015, vol. 2, pp. 115–127.
Pozhidaev, A.E. and Petrova, N.V., Structure of variability of palynomorphological features within and beyond the genus Galeopsis L. Hjl. (Lamiaceae) in the context of divergent morphological evolution, Biol. Bul. Rev., 2022, vol. 13, no. 1, pp. 63–80.
Pozhidaev, A.E., Grigoryeva, V.V., and Semenov, A.N., Structure of individual variability of palynomorphological characteristics of the genus Cestrum (Solanaceae Juss.). Typical form and deviations (morphoses), Zh. Obshch. Biol., 2024 (in press).
Rautian, A.S., Apologia of the comparative method: On the nature of typological knowledge, in Gomologii v botanike: opyt i refleksiya (Homologies in Botany: Experience and Reflection), Oskol’skii, A.A., Sokolov, D.D., and Timonin, A.K., Eds., St. Petersburg: Sankt-Peterburg. Soyuz Uchenykh, 2001, pp. 73–80.
Sheludyakova, M.B., Grigoryeva, V.V., and Pozhidaev, A.E., Morphology of pollen grains of representatives of the genus Scrophularia (Scrophulariaceae), Bot. Zh., 2017, vol. 102, no. 3, pp. 361–379.
Shishova, M., Puzanskiy, R., Gavrilova, O., Kurbanniazov, S., Demchenko, K., et al., Metabolic alterations in male-sterile potato as compared to male-fertile, Metabolites, 2019, vol. 9, no. 2, p. 24. https://doi.org/10.3390/metabo9020024
Stafford, P. and Knapp, S., Pollen morphology and systematics of the zygomorphic flowered nightshades (Solanaceae; Salpiglossideae sensu D’Arcy, 1978 and Cestroideae sensu D’Arcy, 1991, pro parte): A review, Syst. Biodiversity, 2006, vol. 4, no. 2, pp. 173–201. https://doi.org/10.1017/S1477200005001787
Tate, J.A., Acosta, M.C., McDill, J., Moscone, E.A., Simpson, B.B., and Cocucci, A.A., Phylogeny and character evolution in Nierembergia (Solanaceae): Molecular, morphological, and cytogenetic evidence, Syst. Bot., 2009, vol. 34, no. 1, pp. 198–206. https://doi.org/10.1600/036364409787602249
van Campo, M., Patterns of pollen morphological variation within taxa, in The Evolutionary Significance of the Exine, Ferguson, I.K. and Muller, J., Eds., London: Academic Press, 1976, pp. 125–137.
Walker, J.W. and Doyle, J.A., The bases of angiosperms phylogeny: Palynology, Ann. Miss. Bot. Gard., 1975, vol. 62, no. 3, pp. 664–723. https://doi.org/10.2307/2395271
Wodehouse, R.P., Pollen Grains: Their Structure, Identification and Significance in Science and Medicine, New York: McGraw-Hill, 1935.
Funding
This work was performed on the equipment of the Center for Shared Use Cellular and Molecular Technologies for the Study of Plants and Fungi of the Komarov Botanical Institute, Russian Academy of Sciences (St. Petersburg), as part of the state assignment on the topic “Structural and Functional Bases of Development and Adaptation of Higher Plants” (AAA-A18-118031690084-9).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by A. Deryabina
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
APPENDIX А
APPENDIX А
Table A1. Studied samples and brief descriptions of pollen grains of Nierembergia and Bouchetia species
Studied sample | Polar axis, µm | Equatorial diameter, µm | Mesocolpium width, µm | Exine thickness, µm | Sculpture | Distribution unit | Illustrations |
|---|---|---|---|---|---|---|---|
Nierembergia angustifolia Kunth. Mexico. State of Michoacan. C.G. Pringle 414. 21.07.1892 | 37.6–45.9 41.8 ± 4.1 | 51.7–54.6 53.2 ± 1.4 | 38.0–40.2 39.1 ± 2.3 | 1.9–2.1 2.0 ± 3.1 | The sculpture is striated, the striae are short (3.5–5.0 µm), rather wide (0.5–0.6 µm), with rounded ends, tightly adjacent to each other; occasionally, there are short narrow bridges between the jets | Tetrad | |
N. aristata D. Don. Uruguay. P. Lorentz 10.1878 | 45.7–50.8 48.3 ± 3.4 | 48.5–51.8 50.2 ± 2.3 | 37.9–39.6 39.7 ± 1.8 | 2.5–2.7 2.6 ± 0.3 | The sculpture is striated, the striae are long, not wide (0.5–0.6 µm), closely adjacent to each other; with transverse bridges and rare single rounded perforations | Monad | |
N. frutescens Dur. Schvapp. s. n. 30.8.01 | 42.0–50.0 46.0 ± 3.6 | 45.2–49.7 47.5 ± 4.8 | 33.3–36.6 35.0 ± 2.3 | 2.1–2.3 2.2 ± 1.2 | The sculpture is striated, the striae are meridional, long, not wide (0.3–0.5 µm), tightly adjacent to each other | Monad | Figs. 2а–2f |
N. gracilis Hook. Argentina, Tucuman. C.A. O’Donell. 1392. Oct. 1941 | 46.5–53.9 50.2 ± 2.1 | 46.2–50.0 48.1 ± 4.1 | 36.5–38.6 37.6 ± 2.6 | 2.0–2.2 2.1 ± 3.4 | The sculpture is striated, the striae are not wide with bridges, quite tightly adjacent to each other | Monad | Figs. 1а–1f |
N. hippomanica Miers. Argentina, Tucuman. O’Donell 138. 10.1941 | 44.5–50.0 47.3 ± 3.7 | 44.5–54.5 49.5 ± 1.8 | 39.0–40.0 39.5 ± 1.6 | 2.7–3.0 2.9 ± 1.8 | The sculpture is striated, the striae are rather long, not wide (0.3–0.5 µm), tightly adjacent to each other, bifurcating at the ends | Monad | |
N. linarifolia R. Graham. Herder 12961. 3.09.1892 | 40.0–46.0 43.0 ± 3.8 | 46.0–55.0 50.0 ± 2.3 | 40.0–42.8 41.4 ± 1.6 | 2.0 | The sculpture is striated, the striae are rather long, not wide (0.3–0.5 µm), closely adjacent to each other | Monad | |
N. scoparia Sendtn. Brasilia. Sellow. s.n. | 43.6–49.2 46.4 ± 3.8 | 50.0–56.3 53.2 ± 1.3 | 35.8–43.0 49.4 ± 2.8 | 2.0 | The sculpture is striated, the striae are quite long, wide (0.5–1.0 µm), tightly adjacent to each other | Monad | 3k, 3l |
N. stricta Miers. Argentina, Varela 559. 16. 02.1944 | 55.8–58.4 57.1 ± 2.4 | 63.1–72.7 67.9 ± 4.8 | 49.5–50.3 49.9 ± 0.8 | 2.0–2.3 2.2 ± 2.3 | The sculpture is striated, the striae are not long, rather wide (about 1 µm), with rounded ends, some streams have short branches at the ends | Tetrad | |
Bouchetia anomala Britton & Rusby. Flora Texana exsiccate. F. Lindheimer 471. 1846 | 45.2–47.9 46.6 ± 1.8 | 39.5–42.7 41.1 ± 2.7 | 21.1–23.1 22.1 ± 1.9 | 1.8–1.9 | The sculpture is tuberculate-granular, the tubercles (0.23–1.5 µm) are dotted with small granules (about 0.1 µm) | Tetrad | |
B. erecta DC. ex Dun. Mexica, Hidalgo 6912. 23 July 1898 | 45.2–47.9 46.6 ± 3.3 | 45.2–47.9 46.6 ± 3.6 | 21.1–23.1 22.1 ± 2.7 | 1.8–1.9 | The sculpture is coarsely tuberculate and perforated; the tubercles (1.5–5.0 µm) are located at a distance of 1.0–2.5 µm; between them, there are smaller tubercles (less than 1.0 µm); between small tubercles, there are perforations (0.1–0.3 µm) | Tetrad |
Rights and permissions
About this article
Cite this article
Pozhidaev, A.E., Grigoryeva, V.V. & Semyonov, A.N. The Pattern of Natural Variability of Palynomorphological Features by the Example of Some Nierembergia and Bouchetia Species (Solanaceae) and Natural System of Biovariety. Biol Bull Rev 14, 304–319 (2024). https://doi.org/10.1134/S2079086424030083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086424030083