Abstract
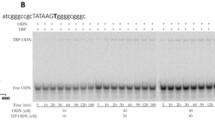
Genetic variability in genes of the circadian clock can manifest itself as a phenotypic variability of physiological functions and behavior, as well as functional disorders not only of the clock but also of other systems leading to the development of pathologies. We analyzed the influence of SNPs localized in the [–70,–20] region from the transcription start site on the promoter affinity of the TATA-binding protein (TBP) in two groups of genes that are components of the human circadian clock system. The first group is comprised of the genes of the core of the circadian oscillator (11 genes); the second comprises the genes of nearest regulatory environment of the circadian oscillator components (21 genes).The control comparison group included genes with other functions (31 genes). The SNP_TATA_Comparator web service was used to predict the effects of the SNPs in the regions of the RNA polymerase II positioning on the TBP/promoter dissociation constant. It was shown that the number of SNP markers reducing the TBP/promoter affinity in the first group of genes is significantly lower than the number of SNP markers increasing affinity (α < 10–3). The opposite was true for the comparison group. A significantly greater number of SNP markers reduced the TBP/promoter affinity than increased it (α < 10–6). Thus, this property may represent a characteristic feature of the genes of the circadian oscillator that may ensure its stability during the genetic variability of the analyzed promoter regions. These predictions are important for identifying the candidate SNP markers for various pathologies associated with the dysfunction of the circadian clock genes for further testing in the experimental and clinical studies, as well as for the verification of the mathematical models of the circadian oscillator.
Similar content being viewed by others
References
Abecasis, G.R., Auton, A., Brooks, L.D., DePristo, M.A., Durbin, R.M., Handsaker, R.E., Kang, H.M., Marth, G.T., and McVean, G.A., An integrated map of genetic variation from 1092 human genomes, Nature, 2012, vol. 491, pp. 56–65. doi 10.1038/nature11632
Abba, M.C., Sun, H., Hawkins, K.A., Drake, J.A., Hu, Y., Nunez, M.I., Gaddis, S., Shi, T., Horvath, S., Sahin, A., and Aldaz, C.M., Breast cancer molecular signatures as determined by SAGE: Correlation with lymph node status, Mol. Cancer Res., 2007, vol. 5, no. 9, pp. 881–890.
Brown, S.A., Kowalska, E., and Dallmann, R., (re)inventing the circadian feedback loop, Dev. Cell, 2012, vol. 22, no. 3, pp. 477–87. doi 10.1016/j.devcel.2012.02.007
Bryant, C.D., Parker, C.C., Zhou, L., Olker, C., Chandrasekaran, R.Y., Wager, T.T., Bolivar, V.J., Loudon, A.S., Vitaterna, M.H., Turek, F.W., and Palmer, A.A., Csnk1e is a genetic regulator of sensitivity to psychostimulants and opioids, Neuropsychopharmacology, 2012, vol. 37, no. 4, pp. 1026–1035. doi 10.1038/npp.2011.287
Burgueno, A.L., Gianotti, T.F., Mansilla, N.G., Pirola, C.J., and Sookoian, S., Cardiovascular disease is associated with high-fat-diet-induced liver damage and up-regulation of the hepatic expression of hypoxiainducible factor 1a in a rat model, Clin. Sci., (London), 2013, vol. 124, no. 1, pp. 53–63. doi 10.1042/CS20120151
Cao, Q., Gery, S., Dashti, A., Yin, D., Zhou, Y., Gu, J., and Koeffler, H.P., A role for the clock gene per1 in prostate cancer, Cancer Res., 2009, vol. 69, no. 19, pp. 7619–7625. doi 10.1158/0008-5472.CAN-08-4199
Chen, L. and Yang, G., PPARs integrate the mammalian clock and energy metabolism, PPAR Res., 2014, vol. 2014, p. 653017. doi 10.1155/2014/653017
Climent, J., Perez-Losada, J., Quigley, D.A., Kim, I.J., Delrosario, R., Jen, K.Y., Bosch, A., Lluch, A., Mao, J.H., and Balmain, A., Deletion of the PER3 gene on chromosome 1p36 in recurrent ER-positive breast cancer, J. Clin. Oncol., 2010, vol. 28, no. 23, pp. 3770–3778. doi 10.1200/JCO.2009.27.0215
Coma, S., Amin, D.N., Shimizu, A., Lasorella, A., Iavarone, A., and Klagsbrun, M., Id2 promotes tumor cell migration and invasion through transcriptional repression of semaphorin 3F, Cancer Res., 2010, vol. 70, no. 9, pp. 3823–3832. doi 10.1158/0008-5472.CAN-09-3048
Fang, L., Yang, Z., Zhou, J., Tung, J.Y., Hsiao, C.D., Wang, L., Deng, Y., Wang, P., Wang, J., and Lee, M.H., Circadian clock gene CRY2 degradation is involved in chemoresistance of colorectal cancer, Mol. Cancer Ther., 2015, vol. 14, no. 6, pp. 1476–1487. doi 10.1158/1535-7163.MCT-15-0030
Flajolet, M., He, G., Heiman, M., Lin, A., Nairn, A.C., and Greengard, P., Regulation of Alzheimer’s disease amyloid- beta formation by casein kinase i, Proc. Natl. Acad. Sci. U.S.A., 2007, vol. 104, pp. 4159–4164.
Frankish, A., Uszczynska, B., Ritchie, G.R., Gonzalez, J.M., Pervouchine, D., Petryszak, R., Mudge, J., Fonseca, N., Brazma, A., Guigo, R., and Harrow, J., Comparison of GENCODE and RefSeq gene annotation and the impact of reference geneset on variant effect prediction, BMC Genomics, 2015, vol. 16, no. 8, p. S2. doi 10.1186/1471-2164-16-S8-S2
Gao, R., Ma, Z., Hu, Y., Chen, J., Shetty, S., and Fu, J., Sirt1 restrains lung inflammasome activation in a murine model of sepsis, Am. J. Physiol. Lung Cell Mol. Physiol., 2015, vol. 308, no. 8, pp. L847–L853. doi 10.1152/ajplung.00274.2014
Gery, S., Komatsu, N., Baldjyan, L., Yu, A., Koo, D., and Koeffler, H.P., The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells, Mol. Cell, 2006, vol. 22, no. 3, pp. 375–382.
Hamzaoui, A., Maalmi, H., Berraies, A., Abid, H., Ammar, J., and Hamzaoui, K., Transcriptional characteristics of CD4 T cells in young asthmatic children: RORC and FOXP3 axis, J. Inflamm. Res., 2011a, vol. 4, pp. 139–146.
Hamzaoui, K., Borhani Haghighi, A., Ghorbel, I.B., and Houman, H., RORC and Foxp3 axis in cerebrospinal fluid of patients with neuro-Behçet’s disease, J. Neuroimmunol., 2011b, vol. 233, nos. 1/2, pp. 249–253. doi 10.1016/j.jneuroim.2011.01.012
Hasan, S., van der Veen, D.R., Winsky-Sommerer, R., Dijk, D.J., and Archer, S.N., Altered sleep and behavioral activity phenotypes in PER3-deficient mice, Am. J. Physiol. Regul. Integr. Comp. Physiol., 2011, vol. 301, no. 6, pp. R1821–R1830. doi 10.1152/ajpregu.00260.2011
Hirano, A., Yumimoto, K., Tsunematsu, R., Matsumoto, M., Oyama, M., Kozuka-Hata, H., Nakagawa, T., Lanjakornsiripan, D., Nakayama, K.I., and Fukada, Y., FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes, Cell, 2013, vol. 152, no. 5, pp. 1106–1118. doi 10.1016/j.cell.2013.01.054
Howroyd, P., Swanson, C., Dunn, C., Cattley, R.C., and Corton, J.C., Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha), Toxicol. Pathol., 2004, vol. 32, no. 5, pp. 591–599. doi 10.1080/01926230490515283
Jilg, A., Lesny, S., Peruzki, N., Schwegler, H., Selbach, O., Dehghani, F., and Stehle, J.H., Temporal dynamics of mouse hippocampal clock gene expression support memory processing, Hippocampus, 2010, vol. 20, no. 3, pp. 377–388. doi 10.1002/hipo.20637
Kasowski, M., Grubert, F., Heffelfinger, C., Hariharan, M., Asabere, A., Waszak, S.M., Habegger, L., Rozowsky, J., Shi, M., Urban, A.E., Hong, M.Y., Karczewski, K.J., Huber, W., Weissman, S.M., Gerstein, M.B., et al., Variation in transcription factor binding among humans, Science, 2010, vol. 328, no. 5975, pp. 232–235. doi 10.1126/science.1183621
Kettner, N.M., Katchy, C.A., and Fu, L., Circadian gene variants in cancer, Ann. Med., 2014, vol. 46, no. 4, pp. 208–220. doi 10.3109/07853890.2014.914808
Kim, J.K. and Forger, D.B., A mechanism for robust circadian timekeeping via stoichiometric balance, Mol. Syst. Biol., 2012, vol. 8, p. 630. doi 10.1038/msb.2012.62
Ko, C.H. and Takahashi, J.S., Molecular components of the mammalian circadian clock, Hum. Mol. Genet., 2006, vol. 15, no. 2, pp. R271–R277.
Korge, S., Grudziecki, A., and Kramer, A., Highly efficient genome editing via CRISPR/Cas9 to create clock gene knockout cells, J. Biol. Rhythms, 2015, vol. 30, no. 5, pp. 389–395. doi 10.1177/0748730415597519
Lin, J.C., Tarn, W.Y., and Hsieh, W.K., Emerging role for RNA binding motif protein 4 in the development of brown adipocytes, Biochim. Biophys. Acta, 2014a, vol. 1843, no. 4, pp. 769–779. doi 10.1016/j.bbamcr.2013.12.018
Lin, J.C., Lin, C.Y., Tarn, W.Y., and Li, F.Y., Elevated SRPK1 lessens apoptosis in breast cancer cells through RBM4-regulated splicing events, RNA, 2014b, vol. 20, no. 10, pp. 1621–1631. doi 10.1261/rna.045583.114
Luo, Y., Wang, F., Chen, L.A., Chen, X.W., Chen, Z.J., Liu, P.F., Li, F.F., Li, C.Y., and Liang, W., Deregulated expression of cry1 and cry2 in human gliomas, Asian Pac. J. Cancer Prev, 2012, vol. 13, no. 11, pp. 5725–5728.
Mehraj, V., Textoris, J., Capo, C., Raoult, D., Leone, M., and Mege, J.L., Overexpression of the Per2 gene in male patients with acute Q fever, J. Infect. Dis., 2012, vol. 206, no. 11, pp. 1768–1770. doi 10.1093/infdis/jis600
Miyazaki, K., Wakabayashi, M., Hara, Y., and Ishida, N., Tumor growth suppression in vivo by overexpression of the circadian component, PER2, Genes Cells, 2010, vol. 15, no. 4, pp. 351–358. doi 10.1111/j.1365-2443.2010.01384.x
Mogno, I., Vallania, F., Mitra, R., and Cohen, B., TATA is a modular component of synthetic promoters, Genome Res., 2010, vol. 20, no. 10, pp. 1391–1397. doi 10.1101/gr.106732.110
Nencioni, A., da Silva, R.F., Fraga-Silva, R.A., Steffens, S., Fabre, M., Bauer, I., Caffa, I., Magnone, M., Sociali, G., Quercioli, A., Pelli, G., Lenglet, S., Galan, K., Burger, F., Vazquez Calvo, S., et al., Nicotinamide phosphoribosyltransferase inhibition reduces intraplaque CXCL1 production and associated neutrophil infiltration in atherosclerotic mice, Thromb. Haemost., 2014, vol. 111, no. 2, pp. 308–322. doi 10.1160/TH13-07-0531
Oishi, K., Ohkura, N., Amagai, N., and Ishida, N., Involvement of circadian clock gene Clock in diabetesinduced circadian augmentation of plasminogen activator inhibitor-1 (PAI-1) expression in the mouse heart, FEBS Lett., 2005, vol. 579, no. 17, pp. 3555–3559.
Oshima, T., Takenoshita, S., Akaike, M., Kunisaki, C., Fujii, S., Nozaki, A., Numata, K., Shiozawa, M., Rino, Y., Tanaka, K., Masuda, M., and Imada, T., Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer, Oncol. Rep., 2011, vol. 25, no. 5, pp. 1439–1446. doi 10.3892/or.2011.1207
Pereira, D.S., van der Veen, D.R., Gonçalves, B.S., Tufik, S., von Schantz, M., Archer, S.N., and Pedrazzoli, M., The effect of different photoperiods in circadian rhythms of per3 knockout mice, Biomed. Res. Int., 2014, vol. 2014, p. 170795. doi 10.1155/2014/170795
Podkolodnaya, O.A., Podkolodnaya, N.N., and Podkolodnyi, N.L., The mammalian circadian clock: Gene regulatory network and computer analysis, Russ. J. Genet., Appl. Res., 2015, vol. 5, no. 4, pp. 354–362.
Podkolodnaya, O.A., Molecular and genetic aspects of interactions of the circadian clock and the energy-producing substrate metabolism in mammals, Russ. J. Genet., 2014, vol. 50, no. 2, pp. 111–122.
Ponomarenko, M., Rasskazov, D., Arkova, O., Ponomarenko, P., Suslov, V., Savinkova, L., and Kolchanov, N., How to use SNP_TATA_Comparator to find a significant change in gene expression caused by the regulatory SNP of this gene’s promoter via a change in affinity of the TATAbinding protein for this promoter, Biomed. Res. Int., 2015, vol. 2015, p. 359835. doi 10.1155/2015/359835
Rasskazov, D.A., Gunbin, K.V., Ponomarenko, P.M., Vishnevskii, O.V., Ponomarenko, M.P., and Afonnikov, D.A., SNP_TATA_Comparator: Web-service for comparing SNPs within the gene promoters associated with human diseases, using the equation of equilibrium binding of the TBP/TATA complex, Vavilovskii Zh. Genet. Sel., 2013, vol. 17, no. 4/1, pp. 599–606.
Reppert, M. and Weaver, D.R., Molecular analysis of mammalian circadian rhythms, Annu. Rev. Physiol., 2001, vol. 63, pp. 647–676.
Rodriguez, N., Yang, J., Hasselblatt, K., Liu, S., Zhou, Y., Rauh-Hain, J.A., Ng, S.K., Choi, P.W., Fong, W.P., Agar, N.Y., Welch, W.R., Berkowitz, R.S., and Ng, S.W., Casein kinase I epsilon interacts with mitochondrial proteins for the growth and survival of human ovarian cancer cells, EMBO Mol. Med., 2012, vol. 4, pp. 952–963. doi 10.1002/emmm.201101094
Sahar, S. and Sassone-Corsi, P., Regulation of metabolism: The circadian clock dictates the time, Trends Endocrinol. Metab., 2012, vol. 23, no. 1, pp. 1–8. doi 10.1016/j.tem.2011.10.005
Sato, F., Kawamura, H., Wu, Y., Sato, H., Jin, D., Bhawal, U.K., Kawamoto, T., Fujimoto, K., Noshiro, M., Seino, H., Morohashi, S., Kato, Y., and Kijima, H., The basic helixloop- helix transcription factor DEC2 inhibits TGF-ß-induced tumor progression in human pancreatic cancer BxPC-3 cells, Int. J. Mol. Med., 2012, vol. 30, no. 3, pp. 495–501. doi 10.3892/ijmm.2012.1037
Savinkova, L.K., Drachkova, I.A., Arshinova, T.V., Ponomarenko, P.M., Ponomarenko, M.P., and Kolchanov, N.A., An experimental verification of the predicted effects of promoter TATA-box polymorphisms associated with human diseases on interactions between the TATA boxes and TATA-binding protein, PLoS One, 2013, vol. 8, no. 2. doi 10.1371/journal.pone.0054626
Sawicka-Gutaj, N., Waligorska-Stachura, J., Andrusiewicz, M., Biczysko, M., Sowinski, J., Skrobisz, J., and Ruchala, M., Nicotinamide phosphorybosiltransferase overexpression in thyroid malignancies and its correlation with tumor stage and with survivin/survivin DEx3 expression, Tumour Biol., 2015, vol. 36, no. 10, pp. 7859–7863. doi 10.1007/s13277-015-3506-z
Shi, Y., Cao, J., Gao, J., Zheng, L., Goodwin, A., An, C.H., Patel, A., Lee, J.S., Duncan, S.R., Kaminski, N., Pandit, K.V., Rosas, I.O., Choi, A.M., and Morse, D., Retinoic acidrelated orphan receptor-a is induced in the setting of DNA damage and promotes pulmonary emphysema, Am. J. Respir. Crit. Care Med., 2012, vol. 186, no. 5, pp. 412–419. doi 10.1164/rccm.201111-2023OC
Toyoshima, M., Howie, H.L., Imakura, M., Walsh, R.M., Annis, J.E., Chang, A.N., Frazier, J., Chau, B.N., Loboda, A., Linsley, P.S., Cleary, M.A., Park, J.R., and Grandori, C., Functional genomics identifies therapeutic targets for MYCdriven cancer, Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 24, pp. 9545–9550. doi 10.1073/pnas.1121119109
Wang, Q., Maillard, M., Schibler, U., Burnier, M., and Gachon, F., Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF, Am. J. Physiol. Regul. Integr. Comp. Physiol., 2010, vol. 299, no. 4, pp. R1013–R1019. doi 10.1152/ajpregu.00241.2010
Wang, T., Yang, P., Zhan, Y., Xia, L., Hua, Z., and Zhang, J., Deletion of circadian gene Per1 alleviates acute ethanolinduced hepatotoxicity in mice, Toxicology, 2013, vol. 314, nos. 2/3, pp. 193–201. doi 10.1016/j.tox.2013.09.009
Watanabe, S., Ageta-Ishihara, N., Nagatsu, S., Takao, K., Komine, O., Endo, F., Miyakawa, T., Misawa, H., Takahashi, R., Kinoshita, M., and Yamanaka, K., SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system, Mol. Brain, 2014, vol. 7, p. 62. doi 10.1186/s13041-014-0062-1
Wilmet, J.P., Tastet, C., Desruelles, E., Ziental-Gelus, N., Blanckaert, V., Hondermarck, H., and Le Bourhis, X., Proteome changes induced by overexpression of the p75 neurotrophin receptor (p75NTR) in breast cancer cells, Int. J. Dev. Biol., 2011, vol. 55, nos. 7-9, pp. 801–809. doi 10.1387/ijdb.113345jw
Wong, V.C., Ko, J.M., Qi, R.Z., Li, P.J., Wang, L.D., Li, J.L., Chan, Y.P., Chan, K.W., Stanbridge, E.J., and Lung, M.L., Abrogated expression of DEC1 during oesophageal squamous cell carcinoma progression is age- and family historyrelated and significantly associated with lymph node metastasis, Br. J. Cancer, 2011, vol. 104, no. 5, pp. 841–849. doi 10.1038/bjc.2011.25
Yamasaki, N., Miyazaki, K., Nagamachi, A., Koller, R., Oda, H., Miyazaki, M., Sasaki, T., Honda, Z.I., Wolff, L., Inaba, T., and Honda, H., Identification of Zfp521/ ZNF521 as a cooperative gene for E2A-HLF to develop acute B-lineage leukemia, Oncogene, 2010, vol. 29, no. 13, pp. 1963–1975. doi 10.1038/onc.2009.475
Yu, S., Matsusue, K., Kashireddy, P., Cao, W.Q., Yeldandi, V., Yeldandi, A.V., Rao, M.S., Gonzalez, F.J., and Reddy, J.K., Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression, J. Biol. Chem., 2003, vol. 278, no. 1, pp. 498–505. doi 10.1074/jbc.M210062200
Yumimoto, K., Akiyoshi, S., Ueo, H., Sagara, Y., Onoyama, I., Ueo, H., Ohno, S., Mori, M., Mimori, K., and Nakayama, K.I., F-box protein FBXW7 inhibits cancer metastasis in a non-cell-autonomous manner, J. Clin. Invest., 2015, vol. 125, no. 2, pp. 621–635. doi 10.1172/JCI78782
Zerbino, D.R., Wilder, S.P., Johnson, N., Juettemann, T., and Flicek, P.R., The Ensembl regulatory build, Genome Biol., 2015, vol. 16, p. 56. doi 10.1186/s13059-015-0621-5
Zhao, H., Zeng, Z.L., Yang, J., Jin, Y., Qiu, M.Z., Hu, X.Y., Han, J., Liu, K.Y., Liao, J.W., Xu, R.H., and Zou, Q.F., Prognostic relevance of Period1 (Per1) and Period2 (Per2) expression in human gastric cancer, Int. J. Clin. Exp. Pathol., 2014, vol. 7, no. 2, pp. 619–630.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.A. Podkolodnaya, D.A. Rasskazov, N.L. Podkolodnyy, N.N. Podkolodnaya, V.V. Suslov, L.K. Savinkova, P.M. Ponomarenko, M.P. Ponomarenko, 2015, published in Vavilovskii Zhurnal Genetiki i Selektsii, 2015, Vol. 19, No. 6, pp. 682–690.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Podkolodnaya, O.A., Rasskazov, D.A., Podkolodnyy, N.L. et al. Effects of SNPs in the positioning regions of RNA polymerase II on the TBP/promoter affinity in genes of the human circadian clock. Russ J Genet Appl Res 6, 759–770 (2016). https://doi.org/10.1134/S207905971607008X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207905971607008X