Abstract
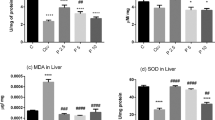
We have studied the age-related features of circadian rhythms of superoxide dismutase (SOD) and catalase activity in the liver of rats under conditions of light deprivation. In standard light conditions (LD), significant daily fluctuations in SOD activity with a maximum at 7:00 a.m. were detected only in young animals (1.5 months) while catalase activity was observed in both young animals (1.5 months) and adults (7.5 months) with peak at 4:00 a.m. The daily dynamics of total and specific activity of SOD and catalase in the liver of young and adult rats differed, depending on the period of ontogeny in which the impact of light deprivation had begun. When females after giving birth and their offspring were moved to darkness (group LD/DD), the circadian rhythms of SOD and catalase activities were found in the young rats and were absent in adult rats. However, circadian rhythms of the antioxidant enzymes (AOE) activities were inherent only in adult rats when light deprivation impacted on pregnant females (group DD/DD). Changes in circadian rhythms under light deprivation were characterized either by a phase shift of the enzymes activity (in LD/DD group) or by a violation of their development in ontogeny (in DD/DD group). With aging a significant decrease of catalase activity was compensated by an increase in the amplitude of circadian rhythms of this enzyme activity in animals of all groups. The presence of an ultradian rhythm in the general circadian cycle characterized by a second peak with a smaller amplitude and shorter period can be considered a distinctive feature of daily fluctuations in AOЕ activity in young rats in LD and LD/DD groups.



Similar content being viewed by others
REFERENCES
Anisimov, V.N., Molekulyarnye i fiziologicheskie mekhanizmy stareniya (Molecular and Physiological Mechanisms of Aging), St. Petersburg: Nauka, 2008, vol. 1.
Rukovodstvo po laboratornym zhivotnym i al’ternativnym modelyam v biomeditsinskikh issledovaniyakh (Manual on Laboratory Animals and Alternative Models in Biomedical Studies), Karkishchenko, N.N. and Grahcev, S.V., Eds., Moscow: Profil’, 2010.
Eticheskaya ekspertiza biomeditsinskikh issledovanii: prakticheskie rekomendatsii (Ethical Expertise of Biomedical Studies: Practical Manual), Belousov, Yu.B., Ed., Moscow: Ross. O-vo Klin. Issled., 2005.
Albarrán, M. T., López-Burillo, S., Pablos, M.I., et al., Endogenous rhythms of melatonin, total antioxidant status and superoxide dismutase activity in several tissues of chick and their inhibition by light, J. Pineal Res., 2001, vol. 30, no. 4, pp. 227–233.
Antolín, I., Rodríguez, C., and Saínz, R.M., Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes, FASEB J., 1996, vol. 10, no. 8, pp. 882–890.
Bass, J. and Takahashi, J.S., Circadian integration of metabolism and energetics, Science, 2010, vol. 330, no. 6009, pp. 1349–1354.
Bears, R.F. and Sizer, I.N., A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase, J. Biol. Chem., 1952, vol. 195, no. 1, pp. 133–140.
Benot, S., Molinero, P., Soutto, M., et al., Circadian variations in the rat serum total antioxidant status: correlation with melatonin levels, J. Pineal Res., 1998, vol. 25, no. 1, pp. 1–4.
Benot, S., Goberna, R., Reiter, R.J., et al., Physiological levels of melatonin contribute to the antioxidant capacity of human serum, J. Pineal Res., 1999, vol. 27, no. 1, pp. 59–64.
Berra, B. and Rizzo, A.M., Melatonin: circadian rhythm regulator, chronobiotic, antioxidant and beyond, Clin. Dermatol., 2009, vol. 27, no. 2, pp. 202–209.
Bonnefont-Rousselot, D. and Collin, F., Melatonin: action as antioxidant and potential applications in human disease and aging, Toxicology, 2010, vol. 278, no. 1, pp. 55–67.
Borjigin, J., Zhang, L.S., and Calinescu, A.A., Circadian regulation of pineal gland rhythmicity, Mol. Cell. Endocrinol., 2012, vol. 349, no. 1, pp. 13–19.
Brooks, E. and Canal, M.M., Development of circadian rhythms: role of postnatal light environment, Neurosci. Biobehav. Rev., 2013, vol. 37, no. 4, pp. 551–560.
Calvo, J. and Boya, J., Postnatal evolution of the rat pineal gland: light microscopy, J. Anat., 1984, vol. 138, no. 1, pp. 45–53.
Christ, E., Korf, H.-W., and von Gall, C., When does it start ticking? Ontogenetic development of the mammalian circadian system, Prog. Brain Res., 2012, vol. 199, pp. 105–118.
Davis, F.C., Melatonin: role in development, J. Biol. Rhythms, 1997, vol. 12, pp. 498–508.
Davis, F.C. and Gorski, R.A., Development of hamster circadian rhythms: role of the maternal suprachiasmatic nucleus, J. Comp. Physiol. A, 1988, vol. 162, no. 5, pp. 601–610.
Deguchi, T., Ontogenesis of a biological clock for serotonin: acetyl coenzyme A N-acetyltransferase in pineal gland of rat, Proc. Natl. Acad. Sci. U.S.A., 1975, vol. 72, no. 7, pp. 2814–2818.
Díaz-Muñoz, M., Hernández-Muñoz, R., Suárez, J., and Chagoya de Sánchez, V., Day-night cycle of lipid peroxidation in rat cerebral cortex and their relationship to the glutathione cycle and superoxide dismutase activity, Neuroscience, 1985, vol. 16, no. 4, pp. 859–863.
Farajnia, S., Deboer, T., and Rohling, J.H., Aging of the suprachiasmatic clock, Neuroscientist, 2014, vol. 20, no. 1, pp. 44–55.
Froy, O., The circadian clock and metabolism, Clin. Sci., 2011, vol. 120, no. 2, pp. 65–72.
Hardeland, R., Coto-Montes, A., and Poeggeler, B., Circadian rhythms, oxidative stress, and antioxidative defense mechanisms, Chronobiol. Int., 2003, vol. 20, no. 6, pp. 921–962.
Hardeland, R., Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance, Endocrine, 2005, vol. 27, no. 2, pp. 119–130.
Husse, J., Eichele, G., and Oster, H., Synchronization of the mammalian circadian timing system: light can control peripheral clocks independently of the SCN clock: alternate routes of entrainment optimize the alignment of the body’s circadian clock network with external time, BioEssays, 2015, vol. 37, no. 10, pp. 1119–1128.
Ikegami, T., Maruyama, Y., Doi, H., et al., Ultradian oscillation in expression of four melatonin receptor subtype genes in the pineal gland of the grass puffer, a semilunar-synchronized spawner, under constant darkness, Front. Neurosci., 2015, vol. 30, pp. 1–10.
Jang, Y.S., Lee, M.H., Lee, S.H., and Bae, K., Cu/Zn superoxide dismutase is differentially regulated in period gene-mutant mice, Biochem. Biophys. Res. Commun., 2011, vol. 409, no. 1, pp. 22–27.
Lacoste, M.G., Ponce, I.T., Golini, R.L., et al., Aging modifies daily variation of antioxidant enzymes and oxidative status in the hippocampus, Exp. Gerontol., 2017, vol. 88, pp. 42–50.
Landgraf, D., Koch, C.E., and Oster, H., Embryonic development of circadian clocks in the mammalian suprachiasmatic nuclei, Front. Neuroanat., 2014, vol. 8, pp. 1–10.
Liu, T. and Borjigin, J., Free-running rhythms of pineal circadian output, J. Biol. Rhythms, 2005, vol. 20, no. 5, pp. 430–440.
Lowry, O.H., Rosenbrough, N.J., Farr, A.L., and Randall, R.J., Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, vol. 193, no. 1, pp. 265–275.
Manikonda, P.K. and Jagota, A., Melatonin administration differentially affects age-induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat liver, Biogerontology, 2012, vol. 13, no. 5, pp. 511–524.
Martin, V., Sainz, R.M., Mayo, J.C., et al., Daily rhythm of gene expression in rat superoxide dismutases, Endocr. Res., 2003, vol. 29, no. 1, pp. 83–95.
Mirmiran, M., Swaab, D.F., Kok, J.H., et al., Circadian rhythms and the suprachiasmatic nucleus in perinatal development, aging and Alzheimer’s disease, Prog. Brain Res., 1992, vol. 93, pp. 151–162.
Misra, H.H. and Fridovich, I., The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase, J. Biol. Chem., 1972, vol. 247, no. 10 P. 3170–3175.
Miyata, R., Tanuma, N., Sakuma, H., and Hayashi, M., Circadian rhythms of oxidative stress markers and melatonin metabolite in patients with xeroderma pigmentosum group A, Oxid. Med. Cell. Longevity, 2016, vol. 2016. doi 10.1155/2016/5741517
Pablos, M.I., Reiter, R.J., Ortiz, G.G., et al., Rhythms of glutathione peroxidase and glutathione reductase in brain of chick and their inhibition by light, Neurochem. Int., 1998, vol. 32, no. 1, pp. 69–75.
Pandi-Perumal, S.R., BaHammam, A.S., Brown, G.M., et al., Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes, Neurotoxic. Res., 2013, vol. 23, no. 3, pp. 267–300.
Pereira, B., Rosa, L.F., Safi, D.A., et al., Hormonal regulation of superoxide dismutase, catalase, and glutathione peroxidase activities in rat macrophages, Biochem. Pharmacol., 1995, vol. 50, no. 12, pp. 2093–2098.
Pevet, P. and Challet, E., Melatonin: both master clock output and internal time-giver in the circadian clocks network, J. Physiol. (Paris), 2011, vol. 105, nos. 4–6, pp. 170–182.
Reiter, R.J., Rosales-Corral, S., Coto-Montes, A., et al., The photoperiod, circadian regulation and chronodisruption: the requisite interplay between the suprachiasmatic nuclei and the pineal and gut melatonin, J. Physiol. Pharmacol., 2011, vol. 62, no. 3, pp. 269–274.
Reiter, R.J., Tan, D.-X., Korkmaz, A., and Rosales-Corral, S.A., Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology, Hum. Reprod. Update, 2014, vol. 20, no. 2, pp. 293–307.
Reppert, S.M., Weaver, D.R., and Rivkees, S.A., Prenatal function and entrainment of a circadian clock, in Research in Perinatal Medicine, Vol. 9: Development of Circadian Rhythmicity and Photoperiodism in Mammals, Reppert, S.M., Ed., Ithaca, NY: Perinatology Press, 1989, ch. 2, pp. 25–44.
Reppert, S.M. and Schwartz, W.J., Maternal coordination of the fetal biological clock in utero, Science, 1983, vol. 220, no. 4600, pp. 969–971.
Rowe, S.A. and Kennaway, D.J., Melatonin in rat milk and the likelihood of its role in postnatal maternal entrainment of rhythms, Am. J. Physiol.-Regul., Integr. Comp. Physiol., 2002, vol. 282, no. 3, pp. R797–R804.
Sancar, A., Lindsey-Boltz, L.A., Kang, T.H., et al., Circadian clock control of the cellular response to DNA damage, FEBS Lett., 2010, vol. 584, no. 12, pp. 2618–2625.
Sani, M., Sebaï, H., Gadacha, W., et al., Catalase activity and rhythmic patterns in mouse brain, kidney and liver, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2006, vol. 145, nos. 3–4, pp. 331–337.
Sanchez, S., Paredes, S.D., Martin, M.I., et al., Effect of tryptophan administration on circulating levels of melatonin and phagocytic activity, J. Appl. Biomed., 2004, vol. 2, no. 3, pp. 169–177.
Sumova, A., Sladek, M., Polidarova, L., et al., Circadian sys tem from conception till adulthood, Prog. Brain Res., 2012, vol. 199, pp. 83–103.
Tan, D.-X., Reiter, R.J., Manchester, L.C., et al., Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger, Curr. Top. Med. Chem., 2002, vol. 2, no. 2, pp. 181–197.
Tapia-Osorio, A., Salgado-Delgado, R., Angeles-Castellanos, M., and Escobar, C., Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat, Behav. Brain Res., 2013, vol. 252, pp. 1–9.
Tomas-Zapico, C., Coto-Montes, A., Martinez-Fraga, J., et al., Effects of continuous light exposure on antioxidant enzymes, porphyric enzymes and cellular damage in the Harderian gland of Syrian hamster, J. Pineal. Res., 2003, vol. 34, no. 1, pp. 60–68.
Wilking, M., Ndiaye, M., Mukhtar, H., and Ahmad, N., Circadian rhythm connections to oxidative stress: implications for human health, Antioxid. Redox Signaling, 2013, vol. 19, no. 2, pp. 192–208.
Xu, Y.-Q., Zhang, D., Jin, T., et al., Diurnal variation of hepatic antioxidant gene expression in mice, PLoS One, 2012, vol. 7, no. 8. doi doi 10.1371/journal.pone.0044237
Yamazaki, S., Yoshikawa, T., Biscoe, E.W., et al., Ontogeny of circadian organization in the rat, J. Biol. Rhythms, 2009, vol. 24, no. 1, pp. 55–63.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Sherstyuk
Rights and permissions
About this article
Cite this article
Khizhkin, E.A., Ilyukha, V.A., Vinogradova, I.A. et al. Circadian Rhythms of Antioxidant Enzyme’s Activity in Young and Adult Rats under Light Deprivation Conditions. Adv Gerontol 8, 328–338 (2018). https://doi.org/10.1134/S2079057018040069
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079057018040069