Abstract
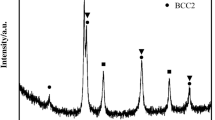
In this study the corrosion behaviour of 17-4 PH (precipitation hardening) stainless steel alloys, produced by adding Ta different amounts with the method of powder metallurgy, and aged at different times was investigated. Four different amounts tantalum (0.15, 0.30, 0.45 and 0.60% weight) was added to the alloy. The prepared powders were produced green compacts at ∅10 × 6 mm dimensions (800 MPa) after mixing with turbule for 2 hours. Green compacts produced were sintered for 1 h at 1300°C under a vacuum of 10–6 mbar. The sintered samples were aged 1, 4 and 8 hours at 480°C after being in solution at 1050°C. Polarization measurements were performed in a corrosion cell, using three different electrode technique. In the corrosion tests, 0.1 M H2SO4 was used as the electrolyte medium. As a result of the studies, the alloy the hardness and densities were increased with increasing Ta amount. Similarly, the hardness and densities of produced samples were with increasing aging time. The corrosion tests revealed that increasing the amount of Ta added to the alloy increased the alloy’s resistance to corrosion. The alloy’s corrosion resistance decreased as aging time increased. The active corrosion mechanism was seen to be pitting corrosion in all alloys.





Similar content being viewed by others
REFERENCES
Mirzadeh, H. and Najafizadeh, A., Mater. Chem. Phys., 2009, vol. 116, no. 1, p. 119. https://doi.org/10.1016/j.matchemphys.2009.02.049
Mirzadeh, H., Najafizadeh, A., and Moazeny, M., Metall. Mater. Trans. A, 2009, vol. 40, no. 12, p. 2950. https://doi.org/10.1007/s11661-009-0029-5
Kochmanski, P. and Nowacki, J., Surf. Coat. Technol., 2008, vol. 202, no. 19, p. 4834. https://doi.org/10.1016/j.surfcoat.2008.04.058
Murayama, M., Hono, K., and Katayama, Y., Metall. Mater. Trans. A, 1999, vol. 30, no. 2, p. 345. https://doi.org/10.1007/s11661-999-0323-2
Nalçacıoğlu, E., Ozyurek, D., and Çetinkaya, K., Trans. Indian Inst. Met., 2019, vol. 72, no. 12, p. 3081. https://doi.org/10.1007/s12666-019-01774-1
Ozyurek, D., Nalcacioglu, E., and Cetinkaya, K., Powder Metall. Met. Ceram., 2020, vol. 59, no. 7, p. 386. https://doi.org/10.1007/s11106-020-00172-3
Bühler, H.E., Gerlach, L., Greven, O., and Bleck, W., Corros. Sci., 2003, vol. 45, no. 10, p. 2325. https://doi.org/10.1016/S0010-938X(03)00062-3
Xiao, M., Li, F., Xie, H., and Wang, Y., Mater. Des., 2012, vol. 34, p. 112. https://doi.org/10.1016/j.matdes.2011.07.065
Gülsoy, H.Ö., Wear, 2007, vol. 262, nos. 3–4, p. 491. https://doi.org/10.1016/j.wear.2006.05.003
Hsiao, C.N., Chiou, C.S., and Yang, J.R., Mater. Chem. Phys., 2002, vol. 74, no. 2, p. 134. https://doi.org/10.1016/S0254-0584(01)00460-6
Wu, Y., German, R.M., Blaine, D., Marx, B., and Schlaefer, C., J. Mater. Sci., 2002, vol. 37, no. 17, p. 3573. https://doi.org/10.1023/A:1016532418920
Kazior, J., Szewczyk-Nykiel, A., Pieczonka, T., Hebda, M., and Nykiel, M., Adv. Mater. Res., 2013, vol. 811, p. 87. https://doi.org/10.4028/www.scientific.net/amr.811.87
Xiao, X., Liu, G., Hu, B., Wang, J., and Ma, W., J. Mater. Sci. Technol., 2015, vol. 31, no. 3, p. 311. https://doi.org/10.1016/j.jmst.2013.04.028
Wang, J., Zou, H., Li, C., Qiu, S.Y., and Shen, B.L., Mater. Charact., 2006, vol. 57, nos. 4–5, p. 274. https://doi.org/10.1016/j.matchar.2006.02.004
Gholipour, A., Shamanian, M., and Ashrafizadeh, F., J. Alloys Compd., 2011, vol. 509, no. 14, p. 4905. https://doi.org/10.1016/j.jallcom.2010.09.216
Wang, J., Zou, H., Li, C., Peng, Y., Qiu, S., and Shen, B., Nucl. Eng. Des., 2006, vol. 236, no. 24, p. 2531. https://doi.org/10.1016/j.nucengdes.2006.03.017
Simsek, I. and Ozyurek, D., Mater. Sci. Eng., C, 2019, vol. 94, p. 357. https://doi.org/10.1016/j.msec.2018.09.047
Özyürek, D., Şimşek, İ., Dincel, Ö., and Şimşek, D., Nevşehir J. Sci. Technol., 2019, vol. 8, p. 98. https://doi.org/10.17100/nevbiltek.633780
Şimşek, D., J. Balıkesir Univ. Inst. Sci. Technol., 2022, vol. 24, no. 1, p. 289. https://doi.org/10.25092/baunfbed.997536
Qin, C., Zhang, W., Nakata, H., Kimura, H., Asami, K., and Inoue, A., Mater. Trans., 2005, vol. 46, no. 4, p. 858. https://doi.org/10.2320/matertrans.46.858
Karaminezhaad, M., Sharafi, S., and Dalili, K., J. Mater. Sci., 2006, vol. 41, no. 11, p. 3329. https://doi.org/10.1007/s10853-005-5416-8
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koçak, S.Ç., Şimşek, D., Şimşek, İ. et al. Corrosion Behaviour of 17-4 PH Stainless Steels Produced by Adding Different Amounts of Alloying Element and Aged at Different Times. Prot Met Phys Chem Surf 59, 1298–1305 (2023). https://doi.org/10.1134/S2070205123701174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205123701174