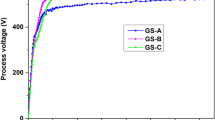
Abstract
In this paper, two major technical problems (growth defects and chromium content loss) encountering when cathodic arc evaporation physical vapor deposition (CAE-PVD) was used for deposition of stainless steel and their subsequent effects on corrosion behavior of the coating in 3.5 wt % NaCl solution have been investigated. Growth defects in spherical and needle-like shapes were the common defects that resulted from CAE-PVD of a stainless steel and played a major role in determining the corrosion behavior of the coating. The results showed a composition difference between the coating matrix (with ~11 at % Cr) and the growth defects, particularly needle-like ones (with ~15 at % Cr). According to SEM images, it seemed that the needle-like defects were passivated and were susceptible to pitting corrosion while coating matrix was corroded. The results also showed that the corrosion of the coating was influenced by two factors: building up micro-galvanic cells between the needle-like defects (as passivated regions) and both coating matrix and the spherical defects (as active sites). In addition, an intense localized corrosion (as micro-crevice corrosion) was observed around the growth defects.
Similar content being viewed by others
References
McCafferty, E., Introduction to Corrosion Science, New York: Springer, 2010.
Asami, K., Hashimoto, K., and Shimodaira, S, Corros. Sci., 1978, vol. 18, p. 151.
Oblonsky, L.J., Ryan, M.P., and Isaacs, H.S, J. Electrochem. Soc., 1998, vol. 145, p. 1922.
Guilemany, J.M., Fernández, J., Espallargas, N., et al, Surf. Coat. Technol., 2006, vol. 200, p. 3064.
Kawakita, J., Fukushima, T., Kuroda, S., et al, Corros. Sci., 2002, vol. 44, p. 2561.
Suegama, P.H., Fugivara, C.S., Benedetti, A.V., et al, Corros. Sci., 2005, vol. 47, p. 605.
Meng, X.M., Zhang, J.B., Han, W., et al, Appl. Surf. Sci., 2011, vol. 258, p. 700.
Adelkhani, H. and Arshadi, M.R., J. Alloys Compd., 2009, vol. 476, p. 234.
Pan, Ch., Liu, L., Li, Y., et al, Electrochim. Acta, 2011, vol. 56, p. 7740.
Liu, L., Li, Y., and Wang, F, Electrochim. Acta, 2010, vol. 55, p. 2430.
Shedden, B.A., Kaul, F.N., Samandi, M., et al, Surf. Coat. Technol., 1997, vol. 97, p. 102.
Poirier, D.M. and Lindfors, P.A, J. Vac. Sci. Technol. A, 1991, vol. 9, p. 278.
Iqbal, Z., Hussain, I., Rauf, A., et al, Prot. Met. Phys. Chem. Surf., 2012, vol. 48, p. 371.
André, A, Surf. Coat. Technol., 1999, vols. 120–121, p.319.
Pauleau, Y. and Barna, P.B., Protective Coatings and Thin Films: Synthesis, Characterization and Applications, Dordrecht, Boston: Kluwer Academic Publ., 1996.
ASTM G 61–86: Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron, Nickel, or Cobalt-Based Alloys, West Conshohocken, PA: ASTM Int., 2003.
Fernandes, C.M., Ferreira, V.M., Senosa, A.M.R., et al, Surf. Coat. Technol., 2003, vol. 176, p. 103.
Song, Y.S., Lee, J.H., Lee, K.H., et al, Surf. Coat. Technol., 2005, vol. 195, p. 227.
André, A, Thin Solid Films, 2010, vol. 518, p. 4087.
Zheng, Z.J., Gao, Y., Gui, Y., et al, Corros. Sci., 2012, vol. 54, p. 60.
Lewis, D.B., Creasey, S.J., Wüstefeld, C., et al, Thin Solid Films, 2006, vol. 503, p. 14.
Wang, H.W., Stack, M.M., and Lyons, S.B, Surf. Coat. Technol., 2000, vol. 126, p. 279.
Kocijan, A., Donik, C., and Jenko, M, Corros. Sci., 2007, vol. 49, p. 2083.
Olsson, C.O.A. and Landolt, D, Electrochim. Acta, 2003, vol. 48, p. 1093.
Li, M., Luo, S., Zeng, C., et al, Corros. Sci., 2004, vol. 46, p. 1369.
Lee, J.B, Mater. Chem. Phys., 2006, vol. 99, p. 224.
Syrett, B.C. and Wing, S.S, Corrosion, 1978, vol. 34, p. 138.
Pan, C., Liu, L., Li, Y, Corros. Sci., 2013, vol. 73, p. 32.
Frankel, G.S, J. Electrochem. Soc., 1998, vol. 145, p. 2186.
Meng, G., Li, Y., and Wang, F, Electrochim. Acta, 2006, vol. 51, p. 4277.
Lakatos-Varshyi, M., Falkenberg, F., and Olefjord, I, Electrochim. Acta, 1998, vol. 43, p. 187.
Igual Munoz, A, Garcia Anton, J., and Guinon, J.L., Corros. Sci., 2007, vol. 49, p. 3200.
Galvele, J.R, Electrochem. Sci. Technol., 1976, vol. 123, p. 464.
Ameer, M.A., Fekry, A.M., and Heakal, F, Electrochim. Acta, 2004, vol. 50, p. 43.
Metikoš-Huković, M., Babić, R., Grubać, Z., et al, Corros. Sci., 2011, vol. 53, p. 2176.
Fattah-alhosseini, A., Saatchi, A., Golozar, M.A., et al, J. Appl. Electrochem., 2010, vol. 40, p. 457.
Fattah-alhosseini, A., Taheri Shoja, S., Heydari Zebardast, B., et al., Int. J. Electrochem., 2011, vol. 2011, Art. ID 152143.
Fattah-alhosseini, A. and Farahani, H, Mater. Sci. Eng., 2013, vol. 10, p. 31.
Freire, L., Carmezim, M.J., Ferreira, M.G.S., et al, Electrochim. Acta, 2011, vol. 56, p. 5280.
Escrivà-Cerdán, C., Blasco-Tamarit, E., García-García, D.M., et al, Electrochim. Acta, 2012, vol. 80, p. 248.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sanati, A., Raeissi, K. & Edris, H. Investigation of the corrosion behavior of cathodic arc evaporated stainless steel coating in 3.5% NaCl. Prot Met Phys Chem Surf 53, 902–909 (2017). https://doi.org/10.1134/S2070205117050197
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205117050197