Abstract
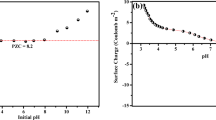
Adsorption of zinc and cadmium ions onto bentonite clays from the Zyryanskoe deposit (Kurgan region, Russia) was studied as a function of contact duration, pH, and temperature. Silanol and aluminol groups of the clay were found to participate in ion exchange and in complex formation with zinc and cadmium ions; a certain process prevails depending on pH. Adsorption of zinc and cadmium ions from equimolar binary solutions was studied. Adsorption of ions onto clay increases with temperature increasing from 293 to 333 K. Adsorption mechanisms of Zn(II) and Cd(II) ions were proposed.
Similar content being viewed by others
References
Mostalygina, L., Elizarova, S., and Kostin, A., Bentonitovye gliny: sorbtsionnye protsessy v prirodookhranitel’nykh tekhnologiyakh (Bentonite Clays: Implementation of Sorption in Environmental Technologies), Saarbrucken: LAP LAMBERT Acad., 2011.
Brindley, G., ICDD Grant-inAid, University Park, PA: Penn State Univ., 1977.
Wu, X.L., Zhao, D., and Yang, S.T., Desalination, 2011, vol. 269, p. 84.
Yan, L., Shan, X., Wen, B., and Owens, G., J. Hazard. Mater., 2008, vol. 156, nos. 1–3, p. 499.
Bhattacharyya, K.G. and Gupta, S.S., Adv. Colloid Interface Sci., 2008, vol. 140, no. 2, p. 114.
Appel, C. and Ma, L., J. Environ. Qual., 2002, vol. 31, p. 581.
Charles, F., et al., The Hydrolysis of Cations, New York: Wiley, 1976.
Zhu J. et al., Chemosphere, 2011, vol. 84, p. 484.
Jolive, J.P., Metal Oxide Chemistry and Synthesis–From Solution to Solid State, New York: Wiley, 2000.
Kopylovich, M.N., et al., J. Mol. Catal. A: Chem., 2003, vol. 206, p. 163.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Kostin, L.V. Mostalygina, O.I. Bukhtoyarov, 2015, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2015, Vol. 51, No. 5, pp. 477–482.
Rights and permissions
About this article
Cite this article
Kostin, A.V., Mostalygina, L.V. & Bukhtoyarov, O.I. The mechanism of adsorption of zinc and cadmium ions onto bentonite clay. Prot Met Phys Chem Surf 51, 773–778 (2015). https://doi.org/10.1134/S2070205115050172
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205115050172