Abstract
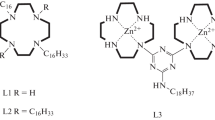
The properties of ultrathin films of two geometric isomers of oligothiophene derivatives containing two crowned styryl fragments in 2- (I) or 3- (II) positions of thiophene rings (Fig. 1) are studied in this work. The ability of these compounds to form stable monolayers at the air/water interface is shown. The structural organization of crown-substituted oligothiophenes in monolayers is determined by the π-π-stacking interaction of hydrophobic styrylthiophene fragments and interaction of hydrophilic macrocycles with the water subphase. Analysis of ultrathin film physicochemical characteristics has shown that the difference in the structure of oligothiophene molecules leads to the formation of distinct monolayer architectures with various electrochemical and optical characteristics. Two types of aggregates (H and J) are generated in monolayers formed from different geometrical isomers at the air/water interface. The effect of barium cation presence in the subphase on the oligomer aggregation in monolayer is discussed. The phase diagrams characterizing the behavior from two-dimensional mixtures of studied crown-substituted oligothiophenes and amphiphilic spreader are plotted basing on compression isotherms accompanied by absorbance and fluorescence spectra of monolayers of different composition. The ability to fine tune the emitted radiation parameters is demonstrated. The obtained results show the efficiency of application of geometrical isomers for investigation of the fundamental “structure-property” ratio of planar supramolecular systems, which is important for organic optics and electronics.
Similar content being viewed by others
References
Freek J. M. Hoeben, Pascal Jonkheijm, E. W. Meijer, and Albertus P. H. J. Schenning, Chem. Rev., 2005, 105(4), pp. 1491–1546.
Wang, C., Dong, H., Hu, W., et al., Chem. Rev., 2012, vol. 112, p. 2208.
Wurthner, F., Angew. Chem., Int. Ed. Engl., 2001, vol. 40, p. 1037.
Katz, H., Dodabalapur, V., Torsi, L., et al., Chem. Mater., 1995, vol. 7, p. 2238.
Fichou, D., Dumarcher, V., and Nunzi, J.-M., Opt. Mater., 1999, vol. 12, p. 255.
Huisman, C.L., Huijser, A., Donker, H., et al., Macromolecules, 2004, vol. 37, p. 5557.
Lim, S.-T. and Shin, D.-M., Synth. Met., 2001, vol. 117, p. 229.
Era, M., Yoneda, S., Sano, T., et al., Thin Solid Films, 2003, vol. 438.
Loi, M.A., Da, ComoE., Dinelli, F., et al., Nat. Mater, 2005, vol. 4, p. 81.
Sullivan, J.T., Harrison, K.E., Mizzell, J.P., et al., Langmuir, 2000, vol. 16, p. 9797.
Singhal, R., Chaubey, A., Kaneto, K., et al., Biotechnol. Bioeng., 2004, vol. 85, p. 277.
Ochiai, K., Rikukawa, M., and Sanui, K., Chem. Commun., 1999, vol. 867.
Dimitrakopolous, C.D. and Malenfant, P.R.L., Adv. Mater., 2002, vol. 14, p. 99.
Gamier, F., Horowitz, G., Peng, X., and Fichou, D., Adv. Mater., 1990, vol. 2, p. 592.
Gamier, F., Hajlaoui, R., Yassar, A., et al., Science, 1994, vol. 1684.
Ostoja, P., Guerri, S., Rossini, S., et al., Synth. Met., 1993, vol. 54, p. 447.
Dodabalapur, A., Torsi, L., and Katz, H., Science, 1995, vol. 268, p. 270.
Brabec, C., Dyakonov, V., Parisi, J., et al., Organic photovoltaics concepts and realization, Heidelberg: Springer-Verlag, 2003.
Vannikov, A.V., Ross. Khim. Zh., 2001, vol. 45, no. 5–6, p. 41.
Simon, J. and Andre, J.-J., Molecular Semiconductors. Berlin: Springer-Verlag, 1985.
Maltsev, E.I., Lypenko, D.A., Brusentseva, M.A., et al., High Energy Chem., 2008, vol. 42, No. 4, p. 67.
Braun, D., Mater. Today, 2002, vol. 5, no. 6, p. 32.
Friend, R.H., Gymer, R.W., Holmes, A.B., et al., Nature, 1999, vol. 397, p. 121.
Yurre, T.A., Rudaya, L.I., Klimova, N.V. et al., Semiconductors, 2003, vol. 37, p. 835.
Geiger, F., Stoldt, M., Schweizer, H., et al., Adv. Mater., 1993, vol. 5, p. 922.
Uchiyama, K., Akimichi, H., Hotta, S., et al., Synth. Met., 1994, vol. 63, p. 57.
Marks, R., Biscarini, F., Zamboni, R., et al., Europhys. Lett., 1995, vol. 32, p. 523.
Horowitz, G., Delannoy, P., Bouchriha, H., et al., Adv. Mater., 1994, vol. 6, p. 752.
Shirota, Y.J., Mater. Chem, 2000, vol. 10, p. 1.
Mitschke, U. and Bauerle, P., Mater. Chem, 2000, vol. 10, p. 1471.
Jousselme, B., Blanchard, P., Levillain, E., et al., Am. Chem. Soc., 2003, vol. 125, p. 1363.
Otsubo, T., Aso, Y., and Takimiya, K., Mater. Chem, 2002, vol. 12, p. 2565.
Lopez-Cabarcos, E., Retama, J., Sholin, V., et al., Polym. Int., 2007, vol. 56, p. 588.
Yassar, A., Gamier, F., Deloffre, F., et al., Adv. Mater., 1994, vol. 6, p. 660.
Roncali, J., Chem. Rev., 1992, vol. 92, p. 711.
Demeter, D., Blanchard, P., Allain, M., et al., Org. Chem, 2007, vol. 72, p. 5285.
Burrell, K., Chen, J., Collis, G., et al., Synth. Met., 2003, vol. 135–136, p. 97.
Si, P., Chi, Q., Li, Z., et al., Am. Chem. Soc., 2007, vol. 129, p. 3888.
Dinelli, F., Murgia, M., Levy, P., et al., Phys. Rev. Lett., 2004, vol. 92, p. 116802.
Murphy, A.R. and Chang, P.C., Vandyke p. et al., Chem. Mater., 2005, vol. 17, p. 6033.
Marsella, M.J. and Swager, T.M.J., Am. Chem. Soc., 1993, vol. 115, p. 12214.
Reitzel, N., Greve, D., Kjaer, K., et al., J. Am. Chem. Soc., 2000, vol. 122, p. 5788.
Nakahara, H., Fukuda, K., Mobius, D., et al., Phys. Chem., 1986, vol. 90, p. 6144.
McRae, E. and Kasha, M., Chem. Phys., 1958, vol. 28, p. 721.
Dimitrakopoulos, C.D. and Mascaro, D.J., IBM J. Res. Dev, 2001, vol. 45, p. 11.
Chen, J., Murphy, A., Esteve, J., et al., Langmuir, 2004, vol. 20, p. 7703.
Ponomarenko, S.A., Borshchev, O.V., Setayesh, S., et al., Organometallics, 2010, vol. 29, p. 4213.
Anokhin, D.V., Defaux, M., Mourran, A., et al., J. Phys. Chem. C, 2012, vol. 116, p. 22727.
Arslanov, V.V., Usp. Khim., 2000, vol. 69, p. 963.
Agina, E.V., Usov, I.A., Borshchev, O.V., et al., Langmuir, 2012, vol. 28, p. 16186.
Sizov, A.S., Agina, E.V., Gholamrezaie, V.V., et al., Appl. Phys. Lett., 2013, vol. 103, p. 4.
Lukovskaya, E., Bobylyova, A., Fedorova, O., et al., Synth. Met., 2007, vol. 157, p. 885.
Lukovskaya, E.V., Bobyleva, A.A., Fedorova, O.A., et al., Russ, Chem. Bull., 2007, vol. 56, p. 932.
Lukovskaya, E.V., Bobyleva, A.A., Fedorova, O.A., et al., Russ, Chem. Bull, 2009, vol. 58, p. 1465.
Lukovskaya, E., Bobylyova, A., Fedorov, Y., et al., Chem. Phys. Chem, 2010, vol. 11, p. 3152.
Stuchebryukov, S.D., Selektor, S.L., Silant’eva, D.A., et al., Prot. Met. Phys. Chem. Surf., 2013, vol. 49, p. 189.
Videlot-Ackermann, C., Ackermann, J., Kawamura K. et al., Org. Electron, 2006, vol. 7, p. 465.
Lednev, I.K. and Petty, M.C., Langmuir, 1994, vol. 10, p. 4185.
Lednev, I.K. and Petty, M.C., J. Phys. Chem., 1994, vol. 98, p. 9601.
Wang, Y., Ozaki, Y., and Iriyama, K., Langmuir, 1995, vol. 11, p. 705.
Zhou, M., Liu, H.L., Yang, H.F., et al., Langmuir, 2006, vol. 22, p. 10877.
Mobius, D., Acc. Chem. Res., 1981, vol. 14, p. 63.
Kuhn, H., Mann, B., Bucher, H., et al., Photogr. Sci. Eng., 1967, vol. 11, p. 233.
Kuhn, H., Pure Appl. Chem., 1979, vol. 51, p. 341.
Bjornholm, T., Greve, D.R., Reitzel, N., et al., J. Am. Chem. Soc., 1998, vol. 120, p. 7643.
Arslanov, V.V., Gorbunova, Yu.G., Selektor, S.L., et al., Russ, Chem. Bull, 2004, p. 2426.
Grauby-Heywang, C., Selektor, S.L., Abraham, E., et al., Prot. Met. Phys. Chem. Surf., 2011, vol. 47, p. 31.
Abraham, E., Selektor, S., Grauby-Heywang, C., and Jonusauskas, G., J. Photochem. Photobiol., vol. 93, p. 44.
Lednev, I.K. and Petty, M.C., Adv. Mater. Opt. Electron, 1994, vol. 4, p. 225.
Xia, C., Locklin, J., Youk, J.H., et al., Langmuir, 2002, vol. 18, p. 955.
Turshatov, A.A., Bossi, M.L., Möbius, D., et al., Langmuir, 2006, vol. 22, p. 1571.
Sergeeva, T.I., et al., Colloids and Surfaces A, 2005, vol. 264, p. 207.
Alfimov, M.V., Herald Russ. Acad. Sci., 2003, vol. 73, p. 429.
Frederick, M. and Fowkes, J., Phys. Chem., 1963, vol. 67, p. 1982.
Khanova, L.A., Evstefeeva, Y.E., and Krishtalik, L.I., Russ. J. Electrochem, 2003, vol. 39, p. 66.
Selektor S., Fedorova, O. Lukovskaya, E., et al., J. Phys. Chem. B, vol. 116, p. 1482.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.L. Selektor, O.A. Fedorova, E.V. Lukovskaya, N.A. Tarasova, O.A. Raitman, A.V. Anisimov, Yu.V. Fedorov, V.V. Arslanov, 2014, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2014, Vol. 50, No. 5, pp. 451–464.
Rights and permissions
About this article
Cite this article
Selektor, S.L., Fedorova, O.A., Lukovskaya, E.V. et al. Planar supramolecular systems based on geometrical isomers of crown-containing oligothiophenes. Prot Met Phys Chem Surf 50, 557–569 (2014). https://doi.org/10.1134/S2070205114050153
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205114050153