Abstract
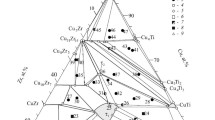
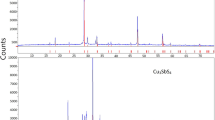
With the use of the substitution solution model, thermodynamic properties of Cu-Ni solid solutions are described in the stratification region. Cross sections of the Cu-Ni-O phase diagram and potentialpH diagrams of the German silver-H2O systems, such as CuNi19-H2O and MNZhMts30-1-1-H2O, at 25°C and 1 bar are plotted. The homogeneity region of NiO x phase at equilibrium with atmospheric oxygen is estimated at various temperatures. Thermodynamic features of the corrosion-electrochemical behavior of copper-nickel alloys are analyzed.
Similar content being viewed by others
References
Smiryagin, A.P., Promyshlennye tsvetnye metally i splavy (Industrial Nonferrous Metals and Alloys), Moscow: Metallurgizdat, 1974.
Novikov, I.I., Medi splavy. Kratkaya khimicheskaya entsiklopediya (Copper Alloys. The Brief Chemical Encyclopedia), Knunyants, I.L., Ed., Moscow: Sovetskaya entsiklopediya, 1964, vol. 3.
Copper-Nickel Alloy Grades, in Marochnik stali i splavov (Steel and Alloy Classifier), http://www.splav.kharkov.com/choose-mat.php?class-id=61.
German Silvers, in Spravochnik Khimika (Chemist’s Handbook), http://chem100.ru/text.php?t=131b.
Cupronickel, http://en.wikipedia.org/wiki/Cupronickel.
Chervyakov, V.I., Markos’yan, G.N., and Pchel’nikov, A.P., Zashch. Met., 2004, vol. 40, no. 2, p. 123.
Kuznetsov, Yu.I. and Rylkina, M.V., Zashch. Met., 2004, vol. 40, no. 5, p. 505.
Markos’yan, G.N., Sirota, D.S., and Pchel’nikov, A.P., Zashch. Met., 2005, vol. 41, no. 4, p. 390.
Sirota, D.S. and Pchel’nikov, A.P., Zashch. Met., 2005, vol. 41, no. 6, p. 652.
Sirota, D.S. and Pchel’nikov, A.P., Zashch. Met., 2005, vol. 41, no. 6, p. 598.
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem. Spravochnik (Phase Diagrams of Binary Metallic Systems), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1997, vol. 2, p. 283.
Phase Diagram. FactSage Database, http://www.crct.polymtl.ca/fact/documentation.
Sabine an Mey, CALPHAD, 1992, vol. 16, no. 3, p. 255.
Straumal, B.B., Protasova, S.G., Mazilkin, et al., J. Mater. Sci., 2012, vol. 47, no. 1, p. 360.
Servant, C., Sundman, B., and Lyon, O., CALPHAD, 2001, vol. 25, no. 1, p. 79.
Sundman, B. and Agren, J., J. Phys. Chem. Solids, 1981, vol. 42, p. 297.
Laptev, D.M., Termodinamika metallurgicheskikh rastvorov (Thermodynamics of Metallurgical Solutions), Chelyabinsk: Metallurgiya, 1992.
Mikhailov, G.G., Leonovich, B.I., and Kuznetsov, Yu.S., Termodinamika metallurgicheskikh protsessov i sistem (Thermodynamics of Metallurgical Processes and Systems), Moscow: Izd. Dom MISiS, 2009.
Tyurin, A.G., Termodinamika khimicheskoi i elektrokhimicheskoi ustoichivosti tverdykh splavov zheleza, khroma i nikelya (Thermodynamics of the Chemical and Electrochemical Resistance of Solid Iron, Chromium, and Nickel Alloys), Chelyabinsk: Izd. ChelGU, 2011.
Tyurin, A.G and Shreiner, A.A., Zashch. Met., 2007, vol. 43, no. 3, p. 313.
Tyurin, A.G., Zashch. Met., 2004, vol. 40, no. 3, p. 256.
Moiseev, G.K., Vatolin, N.A., Marshuk, L.A., and Il’inykh, N.P, Temperaturnye zavisimosti privedennoi energii Gibbsa nekotorykh neorganicheskikh veshchestv: al’ternativnyi bank dannykh ASTRA. OWN (Temperature Dependences of the Reduced Gibbs Energies of Some Inorganic Substances: Alternative ASTRA Database. OWN), Yekaterinburg: UrORAN, 1997.
Sharlai, E.V., Cand. Sci. (Chem.) Dissertation, Chelyabinsk: Izd. YuUrGU, 2008.
Goll, G., Springer Tracts Mod. Phys., 2006, vol. 214, p. 121.
Zakharov, A.Yu., Mitrofanov, V.Ya., and Nikiforov, A.E., Sbornik trudov V Vserossiiskoi nauchnoi konferentsii (Proceedings of the Vth All-Russian Scientific Conf.), Yekaterinburg, 2000, p. 181.
Panov, Yu.D., Zenkov, E.V., and Moskvin, A.S., Sbornik trudov V Vserossiiskoi nauchnoi konferentsii (Proceedings of the Vth All-Russian Scientific Conf.), Yekaterinburg, 2000, p. 366.
Xu, G., J. Superconductivity, 2001, vol. 14, no. 4, p. 509.
Teplov, M.A., Bakharev, O.N., Dooglav, A.V., et al., J. Superconductivity, 1999, vol. 12, no. 1, p. 113.
El-Tantawy, Y.A., El-Kholy, A.E., and Kasem, T.S.E., Corros. Sci., 1978, vol. 18, no. 12, p. 1065.
Muroi, M. and Street, R., Phys. C: Superconductivity, 1995, vol. 248, nos. 3–4, p. 290.
Tokura, Y., Phys. C: Superconductivity, 1991, vols. 185–189, no. 1, p. 174.
Tret’yakov, Yu.D., Khimiya nestekhiometricheskikh okislov (The Chemistry of Nonstoichiometric Oxides), Moscow: Izd. Mosk. Gos. Univ., 1974.
Morachevskii, A.G., Tsemekhman, L.Sh., and Tsymbulov, L.B., Termodinamika sistem i protsessov v metallurgii nikelya i medi (Thermodynamics of Systems and Processes in the Metallurgy of Nickel and Copper), St. Petersburg: Izd. Politekhn. Univ., 2008, issue 12.
Bogatskii, D.P. and Mineeva, I.A, Zh. Obshch. Khim., 1959, vol. 29, no. 4, p. 1382.
Shirokov, Yu.G. and Kirillov, I.P., Izv. Vyssh. Uchebn. Zaved., Khimiya Khim. Tekhnol., 1961, no. 4, p. 599.
Gmelins Handbuch der anorganischen Chemie. 8 Auflage. Nickel. Teil B. (Gmelin’s handbook of inorganic chemistry. 8th edition. Nickel. Part B.), Vienheim: Verlag Chemie, 1966.
Termicheskie konstanty veshcjestv: baza dannykh (Thermal Constants of Substances: Database), http://www.chem.msu.su/cgi-bin/tkv.pl?show=welcome.html.
Tret’yakov, Yu.D., Termodinamika ferritov (Thermodynamics of Ferrites), Leningrad: Khimiya, 1967.
Cotton, F., Advanced Inorganic Chemistry, New York: Wiley, 1972.
Nikolaichuk, P.A., Shalyapina, T.I., Tyurin, A.G., and Mosunova, T.V., Vestn. YuUrGU, Ser. Khimiya, 2010, no. 31 (207), issue 4, p. 72.
Navrotsky, A. and Kleppa, O.J., J. Inorg. Nucl. Chem., 1968, vol. 30, p. 479.
Jacob, K.T., Fitzner, K., and Alcock, C.B., Metallurg. Trans. B, 1977, vol. 8, no. 3, p. 451.
Kulkarni, A.D., Metallurg. Trans., 1973, vol. 4, no. 7, p. 1713.
Samadashvili, I.Dzh., Vararashvili, V.S., Machaladze, T.E., and Pavlenishvili, T.A., Inorg. Mater., 2002, vol. 38, no. 11, p. 1186.
Gollai, A.V., Lykasov, A.A., Pavlovskaya, M.S., and Buldygin, S.V., Russ. J. Phys. Chem., 2006, vol. 80, no. 11, p. 1770.
Khvan, V.A. et al., J. Phase Equil. Diffus., 2011, vol. 32, no. 6, p. 498.
Tyurin, A.G., Zashch. Met., 2000, vol. 36, no. 1, p. 67.
Iwao, K., Watanabe, Y., and Kozuka, Z., Mater. Trans., 1979, vol. 20, no. 10, p. 593.
Schneider, F. and Schmalzried, H., Zeits. Physik. Chem. Neue Folge, 1990, vol. 166, p. 1.
Kjellqvist, L. and Selleby, M., J. Phase Equil. Diffus., 2010, vol. 31, no. 2, p. 113.
Bo, Y. et al., Chin. J. Nonferrous Metals, 2007, vol. 17, no. 10, p. 1705.
Ruzinov, L.P. and Gulyanitskii, B.S., Ravnovesnye prevrashcheniya metallurgicheskikh reaktsii: spravochnik (Equilibrium Transformations in Metallurgical Reactions: Handbook), Moscow: Metallurgiya, 1975.
JANAF Thermochemical Tables, J. Phys. Chem. Ref. Data, 1985, vol. 14, p. 1.
Veryagin, U.D. et al., Termodinamicheskie svoistva neorganicheskikh veshchestv: spravochnik (Thermodynamic Properties of Inorganic Substances: Handbook), Zefirov, A.P., Ed., Moscow: Atomizdat, 1965.
Pankratz, L.B. and Stuve, J.M., and Gokcen, M.A., Thermodynamic Data for Mineral Technology: Handbook, Bureau of Mines, USA, 1984.
Charette, G.G. and Flengas, S.N., J. Electrochem. Soc. Electrochem. Sci., 1968, vol. 115, no. 8, p. 796.
Hugh, St.C.O. and Pownceby, M.I., Contrib. Mineral. Petrol., 1993, vol. 114, no. 3, p. 296.
Wicks, C.E. and Block, F.E., Thermodynamic Properties of 65 Elements: Their Oxides, Halides, Carbides, and Nitrides, Bureau of Mines, USA, 1963.
Elliott, G.F. and Gleiser, M., Thermochemistry for Steelmaking, London: Pergamon, 1960, vol. 1.
Robie, A. and Hemingway, B.S., US Geological Survey Bulletin 2131, Washington: US Government Printing Office, 1995.
Ball, J.W. and Nordstrom, D.K., US Geological Survey. Open-File Rep. No. 91.
Tyurin, A.G., Zashch. Met., 2005, vol. 41, no. 1, p. 74.
Katkov, A.E. and Lykasov, A.A., Inorg. Mater., 2003, vol. 39, no. 2, p. 171.
Park, B.H., Kim, D.-S., Bull. Korean Chem. Soc., 1999, vol. 20, no. 8, p. 939.
Zhuk, N.P., Kurs teorii korrozii i zashchity metallov: uch. posobie dlya vuzov (Textbook on the Theory of Corrosion and Metal Protection. University Course), Moscow: Al’yans, 2006.
Nikolaychuk P.A., Abstracts of the XVIII Int. Conf. on Chemical Thermodynamics in Russia, Samara, 2011, vol. 2, p. 16.
Kireev, V.A., Metody prakticheskikh raschetov v termodinamike khimicheskikh reaktsii (Methods of Practical Calculations in the Thermodynamics of Chemical Reactions), Moscow: Khimiya, 1970.
Nikolaychuk, P.A. and Tyurin, A.G., Abstracts of the XVIII Int. Conf. on Chemical Thermodynamics in Russia, Samara, 2011, vol. 2, p. 17.
Spravochnik khimika (Chemist’s Handbook), Nikol’skii, B.P., Ed., Moscow: Khimiya, 1964, vol. 3.
Kish, L., Kinetics of Electrochemical Metal Dissolution, Budapest: Akademiai Kiado, 1988.
Pourbaix Diagrams, in Substances and Technologies, www.substech.com/dokuwiki/doku.php?id=pourbaixdiagrams.
Nikolaichuk, P.A., Tyurin, A.G., and Kanat’eva, I.I., Mezhvuzovskii sbornik nauchnykh trudov VII Vserossiiskoi konferentsii molodykh uchenykh s mezhdunarodnym uchastiem (Collected Works of the VII All-Russian Conf. Of Young Scientists), Saratov: KUBiK, 2010.
FactSage EpH-Web, http://www.sgte.org/ephweb.php.
THERMEXPERT Potential-pH Diagram Generator, Argentum Solutions, http://www.argentumsolutions.com/cgi-bin/thermexpert.
SUPCRT, Prediction Central, http://www.predcent.org/download/supcrt.
Johnson, J.W., Oelkers, E.H., and Helgeson, H.C., Computers Geosci., 1992, vol. 16, no. 7, p. 899.
The Geochemist’s Workbench (GWB). Rockware, Earth Science and GIS Software, http://www.rockware.com/product/overview.php?id=132.
JNC-TDB, Japan Nuclear Cycle organization, http://migrationdb.jnc.go.jp.
ZZ-HATCHES 19: Database for Radiochemical Modeling, Nuclear Energy Agency, http://www.oecdnea.org/tools/abstract/detail/nea-1210.
PHREEQC-2, http://wwwbrr.cr.usgs.gov/projects/GWC-coupled/phreeqc.
Eriksson, G., Anal. Chim. Acta, 1979, vol. 112, no. 4, p. 375.
SOLGASWATER, http://158.227.5.164/Chemical-Diagrams/html/ISP-Solgaswater.htm.
Atlas of E-pH Diagrams: Intercomparison of Thermodynamic Databases, http://www.gsj.jp/GDB/openfile/files/no0419/openfile419e.pdf.
Tyurin, A.G., Doctoral (Chem.) Dissertation, Chelyabinsk: ChelGU, 2008.
Spravochnik po elektrokhimii (Handbook of Electrochemistry), Sukhotin, A.M., Ed., Leningrad: Khimiya, 1981.
Silverman, D.C., Corrosion, 1981, vol. 37, no. 9, p. 546.
Beverskog, B., Corrosion, 1999, vol. 55, no. 11, p. 1077.
Glasby, G.P., Aquatic Geochem., 1999, vol. 5, no. 3, p. 227.
Uhlig, H.U. and Revie, R.W., Corrosion and Corrosion Control (An Introduction to Corrosion Science and Engineering), New York: Wiley, 1985.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © P.A. Nikolaychuk, A.G. Tyurin, 2012, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2012, Vol. 48, No. 4, pp. 398–412.
Rights and permissions
About this article
Cite this article
Nikolaychuk, P.A., Tyurin, A.G. Thermodynamics of chemical and electrochemical stability of copper-nickel alloys. Prot Met Phys Chem Surf 48, 462–476 (2012). https://doi.org/10.1134/S2070205112040132
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205112040132