Abstract
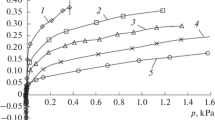
Adsorption isotherms of benzene and adsorption-induced deformation of the AR-V microporous carbon adsorbent in the pressure range from 1 Pa to 14 kPa and at temperatures from 255 to 353 K have been determined. Contraction of the AR-V adsorbent is observed at temperatures of 333 and 353 K in the lowpressure area, which changes into expansion with an increase in pressure. In a low-temperature area (255–313 K), only expansion of the AR-V adsorbent is observed in the whole range of the studied pressures. The value of the maximal relative compression of the adsorbent changes from 0.18 to 0.08%, while the corresponding temperature varies from 353 to 333 K. The maximal relative expansion changes from 0.9 to 0.05% along with an increase in the corresponding temperature from 293 to 353 K.
Similar content being viewed by others
References
Tvardovskiy, A.V., Sorbent Deformation. Amsterdam, Boston, London, etc.: Academic Press / Elsevier, 2006.
Kononyuk, V.F., Sarakhov, A.I., and Dubinin, M.M., Bull. Acad. Sci. USSR, Div. Chem., 1972, no. 8, p. 1691.
Pulin, A.L., Fomkin, A.A., Sinitsyn, V.A., et al., Russ. Chem. Bull., 2001, vol. 50, no. 1, p. 60.
Tvardovski, A.V., Fomkin, A.A., Tarasevich, Y.I., et al., J. Colloid Interface Sci., 1997, vol. 191, p. 117.
Yakovlev, V.Yu., Fomkin, A.A., and Tvardovsi, A.V., J. Colloid Interface Sci., 2003, vol. 268, p. 33.
Ravikovitch, P.I. and Neimark, A.V., Langmuir, 2006, vol. 22, p. 10864.
Ustinov, E.A. and Do, D.D., Carbon, 2006, vol. 44, p. 2652.
Do, D.D., Nicholson, D., and Do, H.D., J. Phys. Chem., 2008, vol. 112, p. 14075.
Fomkin, A.A., Adsorption, 2005, vol. 11, p. 425.
Nabiulin, V.V., Fomkin, A.A., and Tvardovskii, A.V., Prot. Met. Phys. Chem. Surf., 2011, vol. 47, no. 2, p. 162.
Mukhin, V.M., Tarasov, A.V., and Klushin, V.N., Aktivnye ugli Rossii (Activated Carbons in Russia), Moscow: Metallurgiya, 2000.
Dubinin, M.M., Adsorbtsiya i poristost’ (Adsorption and Porosity), Moscow: VAKhZ, 1972.
Vargaftik, N.B., Spravochnik po teplofizicheskim svoistvam gazov i zhidkostei (Handbook on Thermophysical Properties of Gases and Liquids), Moscow: Nauka, 1972.
Shkolin, A.V., Fomkin, A.A., Pulin, A.L., et al., Prib. Tekh. Eksp., 2008, no. 1, p. 163.
Kiselev, A.V. and Kulichenko, V.V., Zh. Fiz. Khim., 1955, vol. 29, p. 663.
Muminov, S., Bering, B.P., Kvlividze, V.I., et al., Dokl. Akad. Nauk, 1966, vol. 169, no. 3, p. 622.
Rusanov, A.I., Kolloidn. Zh., 2007, vol. 69, p. 861.
Serpinskii, V.V. and Yakubov, T.S., Izv. Akad. Nauk SSSR, Ser. Khim., 1981, no. 1, p. 71.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Nabiulin, A.A. Fomkin, A.V. Tvardovskii, 2012, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2012, Vol. 48, No. 4, pp. 333–336.
Rights and permissions
About this article
Cite this article
Nabiulin, V.V., Fomkin, A.A. & Tvardovskii, A.V. Adsorption deformation of a microporous AR-V carbon adsorbent during the adsorption of benzene. Prot Met Phys Chem Surf 48, 398–401 (2012). https://doi.org/10.1134/S2070205112040120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205112040120