Abstract
The growth and production of glacial relic amphipods Monoporeia affinis in a small subarctic lake were studied based on detailed seasonal observations in 2019–2021. Growth and production were closely related to trophic conditions (chlorophyll a concentration). The maximum values were observed at low water temperatures (~5°C) in early summer, coinciding with the spring maximum of chlorophyll. The summer warming of bottom waters was accompanied by a decrease in the growth rate of amphipods, which is apparently associated with the consumption of most of the primary production in the pelagic zone. It is concluded that current climatic conditions can adversely affect glacial relicts even in cold-water lakes of the subarctic zone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The study of the growth and production of aquatic animals has long been one of the main topics of hydrobiological research (Alimov et al., 2013). A particularly large amount of production research was carried out as part of the International Biological Program (Alimov, 1982). However, the bulk of the data was obtained on shallow water bodies in the temperate zone, where the growth of animals occurs at a relatively high temperature during the growing season. There are few studies of cold stenothermal species, in particular, glacial relicts, the life cycle of which occurs at low temperatures (Semenchenko, 1986; Sushchenya et al., 1986). In general, the inhabitants of subarctic and arctic water bodies are still poorly studied (Lento et al., 2019). Currently, the study of the biology of cold-loving species is of particular importance due to climate warming, which is considered a threat to their existence (Penk et al., 2015). This is especially important, given that climate change is more pronounced in subarctic and arctic regions (Vtoroi …, 2014; Heino et al., 2020).
Monoporeia affinis (Lindström) is one of the most interesting components of the fauna of the Holarctic, glacial or glacier–marine relicts, the southern edge of the range of which in general coincides with the boundary of the last glaciation (Sushchenya et al., 1986; Spikkeland et al., 2016; Makhrov et al., 2022). These amphipods are widely distributed in the continental waters of Eurasia, inhabiting numerous lakes and rivers in northern Europe and Siberia, where they often dominate in bottom communities and play an important role in fish feeding (Berezina et al., 2021a, 2021b). The diet of M. affinis is mainly based on phytoplankton settling to the bottom, the development of which is recognized as the leading factor determining the growth and production of these amphipods (Johnson, 1987; Uitto and Sarvala, 1991; Garrison et al., 2022). However, there is not enough information on northern water bodies (especially for the period of freezing), where low temperatures can limit the growth of crustaceans (Arakelova, 2006).
The goal of the present work was to reveal the role of the thermal regime and trophic conditions (Chl a concentration) in a small subarctic lake based on seasonal observations of the growth and production of M. affinis, with special attention to the under-ice period, which was often ignored in production studies.
MATERIALS AND METHODS
This study was performed in the small (0.5 km2) oligotrophic lake Krivoe in northern Karelia (Russia), 30 km south of the Arctic Circle (Biological …, 1975).
The sampling station (66°20.774′ N and 33°37.77′ E) was located in the sublittoral zone of the lake (depth 8.5 m). The station was characterized by a favorable oxygen regime (60–98% saturation). The bottom was covered with brown silty soil.
The structure of the benthic community and interannual changes in the abundance of M. affinis at the studied station in 2002–2019 has been described in previously published works (Maximov, 2021; Maximov et al., 2021). Monoporeia affinis is the dominant species, reaching 31% of the abundance and 60% of the biomass of the total macrozoobenthos, and is used as food by fish living in the lake, perch and vendace (Berezina et al., 2021; Terentiev and Berezina, 2022).
The material was collected from June 2019 to October 2021. During the open water period (June–October), samples were taken monthly and, after the formation of the ice cover, once every 2 months (in December, February, and early April). On each date, five soil samples were taken using Van Veen grab sampler (1/40 m2), which were washed through a sieve with a mesh size of 250 µm. Samples were processed in the laboratory. The crustaceans in the samples were counted, weighed with an accuracy of 0.1 mg using Pioneer PX124 electronic balance (OHAUS Corporation, United States), and fixed in 70% alcohol.
The age of amphipods was estimated from histograms of the size–frequency distribution, which were plotted using microscope and eyepiece micrometer by measuring the body length (distance from the rostrum to the base of the telson) of straightened crustaceans with an accuracy of 0.1 mm. A total of 1282 individuals were measured, i.e., ~60 individuals on each selection date. Due to the short period of replenishment with juveniles (late March–April), the size composition clearly reflected the age structure of the population, and the age groups on the histograms were separated by a pronounced hiatus (Fig. 1).
Histograms of the size-frequency distribution of the population of Monoporeia affinis. (a) Sampling May 26, 2019; (b) June 15, 2019; (c) July 21, 2019; (d) August 25, 2019; (e) September 8, 2019; (f) October 28, 2019; (g) February 17, 2020; (h) April 1, 2020; and (i) May 24, 2020. 0+, 1+, 2+ are selected age groups.
The mass of amphipods was calculated by the equation
where W is the individual wet weight of the crustacean, mg; L is the body length, mm.
The parameters of Eq. (1) were determined based on the measurement and weighing using an analytical balance (accuracy of ± 0.05 mg) of 78 live crustaceans collected in June (47 individuals) and August (31 individuals).
Based on the data, the seasonal dynamics of the growth rate, abundance, and biomass of crustaceans of different ages in 2019–2021 was studied. The specific growth rate was calculated by the equation
where Cw is the average specific growth rate (day-1) for the time interval between the dates of sampling; ln is the natural logarithm; W1 and W2 are the wet weight (mg) of the body of individuals of the same age at the beginning and end of this period, respectively; and ∆t is the duration of the period in days. In the case of a decrease in body weight between observations, which was sometimes observed in the winter period, the growth rate was taken equal to zero.
The biomass of crustaceans was calculated based on the number and average body length of individuals of each age group using Eq. (1). By adding the biomass values of the age groups, the biomass of the entire population was calculated. Since the results of direct weighting strongly correlated (r = 0.97) with the biomass calculated from Eq. (1), only the latter is given in this study.
The production was calculated using the formula (Metody …, 1968):
where P is the production of the cohort for the time interval between samplings; N1 and N2 and W1 and W2 are the number and body weight of crustaceans at the beginning and end of this interval, respectively. For intervals with negative growth, the production and growth rate were taken equal to zero. The production of the entire population was calculated as the sum of the values of the production of individual age groups. When determining the annual production, the initial reference point was timed to the end of May, namely, to the first sampling after the final completion of the period of replenishment of the population with juveniles and the birth of a new generation. The biomass of newborn individuals was considered the generative production of sexually mature amphipods. Thus, the annual production of the population was determined by adding all interval estimates (somatic production) and the biomass of juveniles (generative production) at the end of May. After reaching sexual maturity, amphipods of the family Pontoporeiidae stop growing (Sushchenya et al., 1986). Therefore, when calculating the production of the older age group, breeding amphipods were not taken into account, including only the somatic increase in immature individuals.
Alongside the collection of amphipods, the water temperature was measured and samples were taken to determine the concentration of Chl a. The vertical temperature profile was measured with a CTD probe (MIDAS CTD+, Valeport Ltd., United Kingdom). Since October 2019, in the intervals between field observations, the bottom temperature was recorded with an interval of 2 h using a HOBO Pendant recorder (Onset Computer Corporation, United States) installed 0.5 m from the bottom.
To determine chlorophyll concentration, water samples were taken with a Ruttner bathometer integrally from the surface to the bottom (0–7 m) and 2–3 L of water was filtered through a membrane filter with a pore diameter of ~1 μm. The filters with the precipitate were frozen and stored in a container with silica gel at –20°C until analysis. The Chl a concentration was determined in acetone extract on a UV-1800 spectrophotometer (Shimadzu, Japan) using the method recommended by UNESCO (Determination …, 1966). In separate surveys, measurements were also made with a Cyclops-7 fluorometer installed on a multisensor platform (Turner Designs, United States) to assess the vertical distribution of Chl a concentration.
To assess the effect of environmental factors on growth and production, we calculated the Pearson correlation coefficients between the average values of bottom water temperature, Chl a concentration, specific growth rates, daily population production, and P/B coefficient for the time interval between sampling dates using the STATISTICA 12 software package.
RESULTS
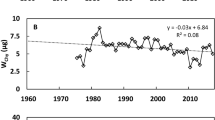
Water temperature and Chl a concentration. A slight increase in the temperature of the bottom water began in spring (Fig. 2) even before the lake was freed from ice in the middle of the 2nd to the beginning of the 3rd decade of May. In June, shortly after ice-cover opening, the surface layer rapidly warmed up, but the temperature of bottom waters remained quite low in the 1st half of summer (Fig. 3), not exceeding 8°C. The maximum temperature of the bottom layer (~10°C) was recorded at the end of August (2021) or in September (2019 and 2020) (Fig. 2).
Vertical distribution of Chl a concentration (a, c, e, g, i, k, m, o) and water temperature (b, d, f, h, j, l, n, p). (a, b), May 26, 2019; (c, d), June 9, 2019; (e, f), July 21, 2019; (g, h), August 24, 2019; (i, j), September 7, 2019; (k, l), October 31, 2019; (m, n), December 26, 2019; and (o, p), March 29, 2020.
Thermal stratification persisted until the end of September–October, when, with the onset of mixing, the entire water column rapidly cooled and homothermy was established (Fig. 3). The periods of freezing of the lake in the studied years differed. In 2019, the lake froze on November 6; in 2020, a stable ice cover formed only by the beginning of December. The late ice formation seems to be associated with a stronger cooling (<2°C) of bottom waters in the winter of 2020–2021 (Fig. 2).
Temperature stratification significantly affected the vertical distribution of Chl a (Fig. 3). Under homothermal conditions in May and October, the distribution of Chl a in the water column was uniform. In summer, the zone of its maximum content approximately corresponded to the metalimnion layer. As stratification weakened, the distribution of Chl a in the water column became more and more uniform (Fig. 3). By the end of winter, when the light conditions for photosynthesis began to improve, an under-ice maximum of Chl a formed, where the pigment concentration sometimes reached almost as high values (≥2 μg/L) as in summer. However, phytoplankton development was limited by a thin layer (~1 m) adjacent to the lower ice surface (Fig. 3).
The seasonal dynamics of Chl a concentration in the 0–7 m layer was characterized by two peaks (Fig. 4). The first was observed at the beginning of summer; the second was at the beginning of autumn. The severity of these peaks varied over the years. In 2019, the maximum concentration (3.2 μg/L) was recorded on June 14; in 2020, the autumn maximum was more pronounced (2.5 μg/L on September 27); in 2021, the summer and autumn peaks almost coincided (~2.1 μg/L) (Fig. 4).
Growth and production of Monoporeia affinis M. affinis is a monocyclic species. After breeding in winter, the crustaceans die. In Lake Krivoe, mating and oviposition occurred in late December–January. The males died shortly after mating: in the February samples, sexually mature males were already absent. The females bore their eggs and died after juveniles hatched in late March–April. The body length of newborn crustaceans was 1.6–1.9 mm. The timing of the release of juveniles from the marsupium in the studied years differed. If on April 1, 2020, almost all females were already free of juveniles, then in 2021, at the time of the survey on April 5, 2021, they were still with embryos. Amphipods grew rapidly in the first summer of their life (age group 0+) until late autumn (Fig. 5). Growth stopped from November, starting again in May of the following year (age 1+).
The age of sexual maturity of M. affinis depends on the size of individuals. In 2019–2021, crustaceans began to breed at a body length of about 6 mm. The great majority of amphipods reached these sizes by the autumn of the second year of life (Fig. 5). In winter, the average body length of crustaceans of the 1+ age group somewhat decreased, which was undoubtedly associated with the reproduction and death of the largest mature individuals. A small number of slowly growing crustaceans did not have time to reach sexual maturity in the 2nd year of life. These crustaceans did not participate in reproduction and survived until the next summer (age 2+); however, their presence in the population was noticeable (2–10% of the total abundance) only in spring and early summer. At a later time, the population was actually represented by two age groups: 0+ and 1+ (Fig. 1), so the growth rate, when studying its seasonal dynamics, was calculated only for these groups.
The data made it possible to track the growth and dynamics of the number of two cohorts from the moment of their birth to puberty. The specific growth rate was maximum at the beginning of the first summer of life and then rapidly decreased (Fig. 6). In winter, crustaceans almost did not grow. A similar picture was repeated in the summer of the following year (age 1+): the growth rate again sharply increased, although it was somewhat lower than in the first year of life.
The number of crustaceans of both generations decreased exponentially over 2 years of life (Fig. 7a). Biomass, in general, showed the opposite trend: its value gradually increased until the start of reproduction in December of the second year of life, although there was some decrease in biomass in winter, which was especially pronounced in the cohort born in 2019 (Fig. 7b). After the end of reproduction, the crustaceans died.
The course of seasonal changes in M. affinis production in the years studied was almost the same. The maximum daily production of the population was observed in early summer. The second, less pronounced, peak took place in autumn (Fig. 8). The average annual values of production, abundance, and biomass during the study period were also quite similar (Table 1).
Influence of environmental factors on growth and production The seasonal dynamics of growth and production almost did not depend on temperature. Although the spring acceleration of growth coincided with a slight increase in water temperature, it began at very low values of the latter (~5°C). The summer heating of bottom waters was accompanied by a decrease in the growth rate of amphipods (Figs. 2, 6).
The influence of Chl a content in water was more significant. Growth and production indicators positively correlated with the average concentration of Chl a in the period between samplings. No statistically significant correlation with water temperature was noted (Table 2). When comparing the data on the seasonal dynamics of Chl a (Fig. 4) and the specific growth rate and production of amphipods (Figs. 6, 8), it was obvious that the autumn maximum of Chl a (especially pronounced in 2020) affected the growth and production noticeably weaker than the summer one. The calculation without taking into account the data for September gave significantly higher correlation coefficients of growth and production indicators with environmental factors (Table 2).
DISCUSSION
As a rule, an intense increase in size in Pontoporeiidae is observed in spring and early summer (Sushchenya et al., 1986; Uitto and Sarvala, 1991). During the summer and autumn, the growth rate decreases rapidly. In shallow (<20 m depth) lakes, growth may accelerate again in early autumn (Johnson, 1987). In Lake Krivoe, seasonal changes in the growth and production of amphipods was consistent with this pattern. In all the years studied, two peaks were clearly distinguished: the first strong peak at the beginning of summer and the second less pronounced one at the beginning of autumn (Figs. 6, 8).
Previously, researchers attributed all spatiotemporal differences in the rate of growth and production of M. affinis solely to the effect of temperature (Grese, 1951). In subsequent years, the opinion about the predominant influence of trophic conditions began to prevail in the literature (Johnson, 1987; Sarvala and Uitto, 1991; Maximov, 2000). The spring growth acceleration of Pontoporeiidae is explained by the increased sedimentation of organic matter after the spring peak of phytoplankton development (Gardner et al., 1990; Uitto and Sarvala, 1991; Lehtonen and Andersin, 1998). The second autumn maximum of amphipod growth is also associated with an increase in the biomass of diatoms in autumn (Johnson, 1987).
In Lake Krivoe, the decrease in the growth rate of M. affinis during the summer occurred against the background of an increase in the temperature of the bottom water. A significant correlation between the growth rate and production rate and temperature (Table 2) was noted only without taking into account the September data (temperature maximum), but it cannot be fully attributed to the effect of the temperature factor, since the temperature and chlorophyll concentration strongly correlated with each other (Table 2).
The seasonal growth dynamics of M. affinis in the lake, on the whole, corresponded to the widely held notion that a powerful influx of fresh detritus into the benthal after the death of spring phytoplankton stimulates the growth of benthic animals (Gardner et al., 1990; Lehtonen and Andersin, 1998; Watkins et al., 2013). The development of phytoplankton in Lake Krivoe began at the end of the freeze-up (March–April), but the development of algae at that time was limited by a very narrow layer of water adjacent to the ice (Fig. 3). It did not noticeably affect either the average concentration of chlorophyll in the water column (Fig. 4) or the production of amphipods (Figs. 6, 8), which almost did not increase. The low growth rate of amphipods in April is apparently partly related to the very low (<3°C) water temperature (Fig. 2). The period of the most intensive growth of amphipods in 2019–2021 was noted in early June along with a sharp increase in the average chlorophyll concentration in the 0–7 m layer. However, the second autumn maximum of chlorophyll content had an extremely weak effect on the growth of amphipods, although, for example, in September 2020 it was much more pronounced than in summer (Fig. 4).
The stimulating effect of spring phytoplankton on benthos is explained by the predominance of diatoms, which are characterized by high sedimentation rates and organic carbon content (Gardner et al., 1990; Watkins et al., 2013). Also, the lack of thermal stratification of the water column in spring contributes to the rapid settling of dying algae to the bottom. However, in Lake Krivoe in spring and autumn, dinophytes dominate (Sharov et al., 2019), which settle to the bottom more slowly than diatoms (Spilling et al., 2018).
Unfortunately, we do not have data on the content of organic substances and photosynthetic pigments directly in bottom sediments, which serve as a more reliable indicator of the food supply of macrozoobenthos compared to the concentration of chlorophyll in the water column (Sigareva, 2012; Timofeeva et al., 2018). Apparently, in the autumn in Lake Krivoe, smaller proportion of primary production reaches the bottom than in spring, because, due to the significant warming of the water column, most of it is mineralized in the pelagial. This probably explains the low rates of growth and production of M. affinis in autumn. In addition, M. affinis, as a species of Arctic origin, apparently has a competitive advantage at low temperatures in early summer, since it has a higher metabolic rate in cold-water conditions compared to boreal species (Berezina et al., 2021). An increase in temperature in autumn, apparently, can increase competition for food with other, more heat-loving benthic animals, such as chironomid larvae. The maximum temperature recorded in the bottom layer (~10°C) of Lake Krivoe was probably unfavorable for amphipods, although, according to experimental data, a temperature of 12–15°C is considered optimal for this species (Kaufman, 2001).
In recent decades, a gradual shift to the north of the southern boundary of the distribution of M. affinis and other glacial relicts has been noted (Sushchenya et al., 1986; Žmudzinski, 1995). Climate warming should be considered one of the probable causes of range reduction. Our data indicate that modern climatic conditions can adversely affect glacial relic amphipods even in cold-water lakes of the subarctic zone. Our results also suggest that the effect of climate warming on M. affinis and other relict species that breed in winter is not limited to the direct impact of higher temperatures. The late freezing of the lake in 2020 led to a strong cooling of the water mass. The bottom water temperature in winter 2020–2021 (1.8°C) was ~1° lower than in the previous winter (Fig. 2), which led to a significant (at least 2-week) shift in the emergence of juveniles from the marsupium. This shift corresponds to the literature data on the duration of embryogenesis in M. affinis at close temperatures. Thus, in the lakes of Norway at a temperature of 2.7°C, the duration of the incubation period is 98 days, and in the Belarusian Lake Yuzhny Volos at 1.4°C, it reaches 115 days (Sushchenya et al., 1986). Climate warming has led to a reduction in the ice period, late freezing, and early opening of lakes in the Northern Hemisphere (Sharma et al., 2021), which, on the one hand, leads to cooling of deep waters and, in turn to an earlier spring development of algae. It can be assumed that, during late freezing in very mild winters, juvenile amphipods will be born after the spring maximum of phytoplankton, which, given the role of spring phytoplankton in amphipod nutrition, will adversely affect their further development. Perhaps this is one of the reasons for the disappearance or sharp decline in the abundance of M. affinis and other winter-breeding relicts in the southern part of their range.
Populations of M. affinis are characterized by cyclic fluctuations in abundance; however, in adjacent years, the abundance and biomass of crustaceans are usually similar (Maximov et al., 2021). In 2019–2021, indicators of amphipod development differed insignificantly (Table 1). A comparison with the results of the 1st study of M. affinis production in Lake Krivoe, conducted in 1968–1969 (Biologicheskaya …, 1975), showed that the production and biomass of amphipods were then approximately two times lower than in 2019–2021. However, data from the 1960s do not go beyond the values observed in the last 20 years.
CONCLUSIONS
The growth and production of glacial relict amphipods M. affinis in Lake Krivoe is closely related to trophic conditions. The maximum indicators were recorded at low temperatures in early summer after the spring peak of phytoplankton development. The summer warming of bottom waters was accompanied by a decrease in the growth rate of amphipods, apparently due to the consumption of most of the primary production in the pelagic zone. Climate warming can adversely affect relict amphipods even in the lakes of the subarctic zone.
REFERENCES
Alimov, A.F., Productivity of macrobenthic invertebrate communities in continental water bodies of the USSR, Gidrobiol. Zh., 1982, vol. 18, no. 2, p. 7.
Alimov, A.F., Bogatov, V.V., and Golubkov, S.M., Produktsionnaya gidrobiologiya (Production Hydrobiology), St. Petersburg: Nauka, 2013.
Arakelova, E.S., Energy metabolism and growth of Monoporeia affinis Lindstr. in the Northern Lake Krivoe (Karelia), Russ. J. Ecol., 2006, vol. 37, p. 167.
Berezina, N., Kalinkina, N., and Maximov, A., Distribution and functional ecology of malacostracan Crustaceans in Russian Northern and Arctic Lakes, in Lake Water: Properties and Uses (Case Studies of Hydrochemistry and Hydrobiology of Lakes in Northwest Russia), New York: Nova Sci., 2021a.
Berezina, N.A., Litvinchuk, L.F., and Maximov, A.A., Relations between the food spectrum of fishes and the composition of zooplankton and benthos in a subarctic lake, Inland Water Biol., 2021b, no. 4, p. 438. https://doi.org/10.1134/S1995082921040052
Biologicheskaya produktivnost' severnykh ozer. 1. Ozera Krivoe i Krugloe (Biological Productivity of Northern Lakes. 1. Lakes Krivoe and Krugloe), Leningrad: Nauka, 1975.
Determination of photosynthetic pigments. Report of SCOR – UNESCO working group 17 on determination of photosynthetic pigments, in Determination of Photosynthetic Pigments in Sea-Water, Paris: UNESCO, 1966.
Gardner, W.S., Quigley, M.A., Fahnenstiel, S.D., and Frez, W.A., Pontoporeia hoyi—a direct trophic link between spring diatoms and fish in Lake Michigan, in Large Lakes. Ecological Structure and Function, Berlin: Springer-Verlag, 1990.
Garrison, J.A., Karlson, A.M.L., and Nascimento, F.J.A., Amphipod isotope composition, condition and reproduction in contrasting sediments: A reciprocal transfer, Front. Mar., 2022, vol. 9. https://doi.org/10.3389/fmars.2022.789700
Greze, V.N., The production of Pontoporeia affinis and a method for its determination, Tr. Vses. Gidrobiol. O-va., 1951, vol. 3, p. 33.
Heino, J., Culp, J.M., Erkinaro, J., et al., Abruptly and irreversibly changing Arctic freshwaters urgently require standardized monitoring, J. Appl. Ecol., 2020, vol. 57, no. 7, p. 1192.
Johnson, R.K., The life history, production and food habits of Pontoporeia affinis Lindström (Crustacea: Amphipoda) in mesotrophic Lake Erken, Hydrobiologia, 1987, vol. 144, p. 277.
Kaufman, B.Z., Preferential behavior of Pontoporeia affinis Lindstrom (Crustacea, Amphipoda), Hydrobiol. J., 2001, vol. 37, no. 2, p. 6.
Lehtonen, K.K. and Andersin, A.-B., Population dynamics, response to sedimentation and role in benthic metabolism of the amphipod Monoporeia affinis in an open-sea area of the Northern Baltic Sea, Mar. Ecol. Prog. Ser., 1998, vol. 168, p. 71.
Lento, J., Goedkoop, W., Culp, J., et al., State of the Arctic Freshwater Biodiversity, Akureyri: Conserv. Arct. Flora Fauna Internat. Sec., 2019.
Makhrov, A.A., Bolotov, I.N., Vinarski, M.V., et al., Origin of glacial relicts in Northern and Central Europe: Four waves of introduction of gold-water species from Asia (Review), Inland Water Biol., 2022, vol. 15, no. 6, p. 707. https://doi.org/10.31857/S0320965222060146
Maximov, A.A., Role of Monoporeia affinis (Lindström) (Crustacea; Amphipoda) in benthic communities of the eastern part of the Gulf of Finland, Extended Abstract of Cand. Sci. Dissertation, St. Petersburg, 2000.
Maximov, A.A., Population dynamics of the glacial relict amphipods in a subarctic lake: role of density-dependent and density-independent factors, Ecol. Evol., 2021, vol. 11, no. 22, p. 15905. https://doi.org/10.1002/ece3.8260
Maximov, A.A., Berezina, N.A., and Maximova, O.B., Interannual changes in benthic biomass under climate-induced variations in productivity of a small northern lake, Fundam. Appl. Limnol., 2021, vol. 194, no. 3, p. 187. https://doi.org/10.1127/fal/2020/1291
Metody opredeleniya produktsii vodnykh zhivotnykh (Methods for Determining Aquatic Animal Products), Minsk: Vysheishaya Shkola, 1968.
Penk, M., Donohue, I., Récoules, V., and Irvine, K., Elevated temperatures interact with habitat quality to undermine survival of ectotherms in climatic refugia, Diversity Distrib., 2015, vol. 21, no. 2, p. 200.
Sarvala, J. and Uitto, A., Production of the benthic amphipods Pontoporeia affinis and P. femorata in a Baltic archipe-lago, Ophelia, 1991, vol. 34, no. 2, p. 71.
Semenchenko, V.P., Production and energy flow through the population of the relict amphipod Pontoporeia affinis Lindström in Lake South Volos, Gidrobiol. Zh., 1986, vol. 22, no. 3, p. 29.
Sharma, S., Richardson, D.C., Woolway, R.I., et al., Loss of ice cover, shifting phenology, and more extreme events in Northern Hemisphere Lakes, J. Geophys. Res.: Biogeosci., 2021, vol. 126, no. 10, p. e2021JG006348.
Sharov, A.N., Nikulina, V.N., and Maximov, A.A., Phytoplankton of a subarctic lake under climatic variability, Reg. Ekol., 2019, vol. 2, no. 56, p. 51.
Sigareva, L.E., Khlorofill v donnykh otlozheniyakh volzhskikh vodoemov (Chlorophyll in the Bottom Sediments of the Volga Reservoirs), Moscow: KMK, 2012.
Spikkeland, I., Kinsten, B., Kjellberg, G., et al., The aquatic glacial relict fauna of Norway—an update of distribution and conservation status, Fauna Norv., 2016, vol. 36, p. 51.
Spilling, K., Olli, K., Lehtoranta, J., et al., Shifting diatom—dinoflagellate dominance during spring bloom in the Baltic Sea and its potential effects on biogeochemical cycling, Front. Mar. Sci., 2018, vol. 5, no. 327, p. 1. https://doi.org/10.3389/fmars.2018.00327
Sushchenya, L.M., Semenchenko, V.P., and Vezhnovets, V.V., Biologiya i produktsiya lednikovykh reliktovykh rakoobraznykh (Biology and Production of Glacial Relict Crustaceans), Minsk: Nauka Tekh., 1986.
Terentjev, P.M. and Berezina, N.A., Ecological and morphological characteristics and feeding of perch (Perca fluviatilus Linnaeus, 1758) in the autumn-winter period in dystrophic and oligotrophic lakes of northern Karelia (Russia), Inland Water Biol., 2022, vol. 15, no. 6, p. 915. https://doi.org/10.1134/S1995082922060177
Timofeeva, N.A., Perova, S.N., and Sigareva, L.E., Distribution of sedimentary pigments and macrozoobenthos in the deepwater part of the Rybinsk Reservoir, Contemp. Probl. Ecol., 2018, vol. 11, p. 652.
Uitto, A. and Sarvala, J., Seasonal growth of benthic amphipods Pontoporeia affinis and P. femorata in a Baltic archipelago in relation to environmental factors, Mar. Biol., 1991, vol. 111, p. 237.
Vtoroi otsenochnyi doklad Rosgidrometa ob izmeneniyakh klimata i ikh posledstviyakh na territorii Rossiiskoi Federatsii (The Second Assessment Report of Roshydromet on Climate Change and its Consequences on the Territory of Russian Federation), Moscow: Rosgidromet, 2014.
Watkins, J., Rudstam, L., Crabtree, D., and Walsh, M., Is reduced benthic flux related to the Diporeia decline? Analysis of spring blooms and whiting events in Lake Ontario, J. Great Lakes Res., 2013, vol. 39, p. 395.
Žmudzinski, L., Retreat of Pallasiola quadrispinosa (G.O. Sars) and Monoporeia affinis (Lindström) from the Polish lakes, Pol. Arch. Hydrobiol., 1995, vol. 42, no. 4, p. 401.
ACKNOWLEDGMENTS
We thank N.A. Berezina, D.M. Martynova, and V.V. Smirnov (Zoological Institute, Russian Academy of Sciences); P.M. Terentiev (Institute of Problems of Industrial Ecology of the North, Kola Science Center of the Russian Academy of Sciences); and A.N. Sharov (Papanin Institute of Biology of Inland Waters, Russian Academy of Sciences).
Funding
This work was performed at the White Sea Biological Station of the Zoological Institute of the Russian Academy of Sciences with financial support from the Ministry of Education and Science of the Russian Federation, project nos. 122031100274-7 and 122031100283-9, and the Russian Foundation for Basic Research, grant no. 19-04-01000.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Bulaev
Abbreviations: Chl a, chlorophyll a.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maximov, A.A., Maximova, O.B. & Usov, N.V. Seasonal Dynamics of Growth and Production of Monoporeia affinis (Amphipoda: Pontoporeiidae) in a Subarctic Lake: The Role of Temperature and Trophic Conditions. Inland Water Biol 16, 912–922 (2023). https://doi.org/10.1134/S1995082923050103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082923050103