Abstract
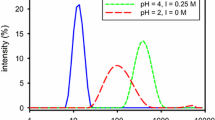
The possibility of preparing gold dispersions with a narrow and controlled size distribution of nanoparticles in the process of a UV-induced reduction of Au3+ of HAuCl4 in chitosan solutions by varying the polysaccharide macromolecule conformation is shown. Solutions with a spiral conformation of chitosan macromolecules are characterized by the formation of Au nanoparticles with smaller average sizes \( \bar l \) = 2 nm and a narrower distribution from 1 to 7 nm, when compared to the conformation of a statistical coil (\( \bar l \) = 5 nm; from 2 to 14 nm, respectively). The particle shape in both cases is close to spherical. Nanodispersions are aggregately stable for no less than 160 days.
Similar content being viewed by others
References
M.-C. Daniel and D. Astruc, “Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology,” Chem. Rev. 104(1), 293–346 (2004).
H. Huang and X. Yang, “Synthesis of Chitosan-Stabi-lized Gold Nanoparticles in the Absence/Presence of Tripolyphosphate,” Biomacromolecules 5(6), 2340–2346 (2004).
H. Huang, Q. Yuan, and X. Yang, “Preparation and Characterization of Metal—Chitosan Nanocomposites,” Colloids Surf., B 39(1-2), 31–37 (2004).
H. Huang and X. Yang, “Synthesis of Polysaccharide-Stabilized Gold and Silver Nanoparticles: A Green Method,” Carbohydr. Res. 339(15), 2627–2631 (2004).
M. R Kasaai, “Calculation of Mark—Houwink—Sakurada (MHS) Equation Viscometric Constants for Chitosan in Any Solvent-Temperature System Using Experimental Reported Viscometric Constants Data,” Carbohydr. Polym. 68(3), 477–488 (2007).
E. N. Fedoseeva, F. A. Smirnova, and V. B. Fedoseev, “Viscous Properties of Solutions of Chitosan and Its Reactivity,” Vestn. Nizhegorodsk. Univ., Ser. Khim., No. 4, 59–64 (2008).
J. D. M. Rinaudo, G. Pavlov, and J. Desbriéres, “Influence of Acetic Acid Concentration on the Solubilization of Chitosan,” Polymer 40(25), 7029–7032 (1999).
S. Fink and M. A. El-Sayed, “Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods,” J. Phys. Chem. B 103(7), 8410–8426 (1999).
K. R Brown, D. G. Walter, and M. J. Natan, “Seeding of Colloidal Au Nanoparticle Solutions: 2. Improved Control of Particle Size and Shape,” Chem. Mater. 12(2), 306–313 (2000).
A. Guinier, X-ray Crystallographic Technology (Hilger and Watts, Fondon, 1952; Fizmatgiz, Moscow, 1961).
G. Famarque, J.-M. Fucas, C. Viton, and A. Domard, “Physicochemical Behavior of Homogeneous Series of Acetylated Chitosans in Aqueous Solution: Role of Various Structural Parameters,” Biomacromolecules 6(1), 131–142 (2005).
C. Tanford, Physical Chemistry of Macromolecules (Wiley, New York, 1961; Khimiya, Moscow, 1965).
S. R. Rafikov, S. A. Pavlova, and I. I. Tverdokhlebova, Methods for Determining Molecular Weights and Polydis-persity of Macromolecular Compounds (Academy of Sciences of the Soviet Union, Moscow, 1963) [in Russian].
D. F Svergun and F. F Feigin, Structure Analysis by Small-Angle X-Ray and Neutron Scattering (Nauka, Moscow, 1986; Plenum, New York, 1987).
G. Porod, “Die Röntgenkleinwinkelstreuung von dichtgepackten kolloiden Systemen: I,” Kolloid. Z. 124(2), 83–114 (1951).
O. E. Fitmanovich, G. V. Marmuzov, A. A. Fitmanovich, and I. M. Papisov, “Selectivity of Interactions between Copper Nanoparticles and Macromolecules of Polyelectrolyte and Nonionogenic Polymers,” Vysoko-mol. Soedin, Ser. A. 45(9), 1533–1543 (2003) [Polym. Sci., Ser. A 45 (9), 906–914 (2003)].
F. Huang, M. Zhai, J. Peng, F. Xu, J. Fi, and G. Wei, “Synthesis, Size Control, and Fluorescence Studies of Gold Nanoparticles in Carboxymethylated Chitosan Aqueous Solutions,” J. Colloid Interface Sci. 316(2), 398–404 (2007).
S. Fink and M. A. El-Sayed, “Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles,” J. Phys. Chem. B 103(21), 4212–4217 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.A. Smirnova, T.A. Gracheva, A.E. Mochalova, T.A. Kuz’micheva, E.N. Fedoseeva, 2010, published in Rossiiskie nanotekhnologii, 2010, Vol. 5, Nos. 1–2.
Rights and permissions
About this article
Cite this article
Smirnova, L.A., Gracheva, T.A., Mochalova, A.E. et al. Peculiarities of gold nanoparticle formation in chitosan solutions doped with HAuCl4 . Nanotechnol Russia 5, 78–82 (2010). https://doi.org/10.1134/S1995078010010088
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078010010088