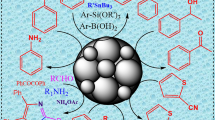
Abstract
This review considers the interrelation between homogeneous, heterogeneous, and nanosized catalytic systems for carrying out reactions in liquid phase (organic solvents, water, ionic liquids, and melts). Most attention is drawn to describing the process of atoms leaching from the surface of nanoparticles and its influence on the activity and selectivity of the catalysts. Well-known reactions of cross-coupling, the data for which have been obtained using all types of catalytic samples, are taken as an example. The area where cross-coupling reactions in fine organic synthesis can be applied includes pharmaceutical chemistry and medical applications of organic chemistry, the production of Pharmaceuticals and biologically active compounds, the chemistry of dyes and organic functional materials, and a number of industrially important processes. The implementation of highly effective nanosized catalysts in this area is expected to provide conceptual changes which can solve many important problems.
Similar content being viewed by others
References
A. L. Buchachenko, “Nanochemistry: A Direct Route to High Technologies of the New Century,” Usp. Khim. 72, 419–437 (2003).
M. D. Cobb and J. Macoubrie, “Public Perceptions about Nanotechnology: Risks, Benefits, and Trust,” J. Nanopart. Res. 6, 395–405 (2004).
Nanotechnology Research Directions: Vision for Nanotechnology in the Next Decade, Ed. by M. C. Roco, R. S. Williams, and P. Alivisatos (Kluwer, Dordrecht, The Netherlands, 2000; Mir, Moscow, 2002).
G. B. Sergeev, Nanochemistry (Elsevier, Amsterdam, 2006; Moscow State University, Moscow, 2007).
A. I. Gusev and A. A. Rempel, Nanocrystalline Materials (Cambridge International Science Publishing, Cambridge, 2004).
V. N. Parmon, “Thermodynamic Analysis of the Effect of the Nanoparticle Size of the Active Component on the Adsorption Equilibrium and the Rate of Heterogeneous Catalytic Processes,” Dokl. Akad. Nauk 413(sn1), 53–59 (2007) [Dokl. Phys. Chem. 413 (1), 42–48 (2007)].
T. N. Rostovshchikova, V. V Smirnov, V. M. Kozhevin, D. A. Yavsin, and S. A. Gurevich, “Intercluster Interactions in Catalysis with Nanometer-Sized Metal Particles,” Ross. Nanotekhnol. 2(1–2), 47–60 (2007).
A. Yu. Stakheev and L. M. Kustov, “Effects of the Support on the Morphology and Electronic Properties of Supported Metal Clusters: Modern Concepts and Progress in 1990s,” Appl. Catal. 188, 3–35 (1999).
A. Yu. Stakheev, Yu. M. Shulga, N. A. Gaidai, N. S. Telegina, L. M. Kustov, and K. M. Minachev, “New Evidence for the Electronic Nature of the Strong Metal-Supported Interaction Effect over a Pt/TiO2 Hydrogenation Catalyst,” Mendeleev Commun. 11, 186–188(2001).
A. S. Lisitsyn, V. N. Parmon, V. K. Duplyakin, and V. A. Likholobov, “Modern Problems and Prospects for the Development of Investigations in the Field of Preparation of Deposited Palladium Catalysts,” Ross. Khim. Zh. 50(4), 140–153 (2006).
I. I. Moiseev and M. N. Vargaftik, “Clusters and Colloidal Metals in Catalysis,” Zh. Obshch. Khim. 72(4), 550–560 (2002) [Russ. J. Gen. Chem. 72 (4), 512–523 (2002)].
V. I. Bukhtiyarov and M. G. Slin’ko, “Metallic Nanosystems in Catalysis,” Usp. Khim. 70, 167–181 (2001).
S. A. Nikolaev, V. V. Smirnov, I. P. Beletskaya, A. Yu. Vasil’kov, A. V. Naumkin, and F. A. Tyurina, “Synergism of the Catalytic Action of Au—Ni Nanocomposites in the Course of Allyl Isomerization of Allylbenzene,” Ross. Nanotekhnol. 2(9–10), 58–67 (2007).
A. Solinas and M. Taddei, “Solid-Supported Reagents and Catch-and-Release Techniques in Organic Synthesis,” Synthesis, No. 16, 2409–2453 (2007).
Metal-Catalyzed Cross-Coupling Reactions, Ed. by A. de Meijere and F. Diederich (Wiley, Berlin, 2004).
Catalytic Heterofunctionalization, Ed. by A. Togni and H. Grützmacher (Wiley, Weinheim, 2001).
Applied Homogeneous Catalysis with Organometallic Compounds, Ed. by B. Cornils and W. A. Herrmann, 2nd ed. (Wiley, Weinheim, 2002).
A. R Tao, S. Habas, and P. D. Yang, “Shape Control of Colloidal Metal Nanocrystals,” Small 4, 310–325 (2008).
A. Roucoux, J. Schulz, and H. Patin, “Reduced Transition-Metal Colloids: A Novel Family of Reusable Catalysts?” Chem. Rev. 102, 3757–3778 (2002).
B. F. Cushing, V. F. Kolesnichenko, and C. J. O’Connor, “Recent Advances in the Fiquid-Phase Syntheses of Inorganic Nanoparticles,” Chem. Rev. 104, 3893–3946 (2004).
H. Bönnemann and R. M. Richards, “Nanoscopic Metal Particles-Synthetic Methods and Potential Applications,” Eur. J. Inorg. Chem. 2455–2480 (2001).
J. P. Wilcoxon and B. F. Abrams, “Synthesis, Structure, and Properties of Metal Nanoclusters,” Chem. Soc. Rev. 35, 1162–1194 (2006).
Handbook of Heterogeneous Catalysis, Ed. by G. Ertl, G. Ertl, H. Knözinger, F. Schüth, and J. Weitkamp, 2nd ed. (Wiley Weinheim, Germany, 2008).
F. D. Pachon and G. Rothenberg, “Transition-Metal Nanoparticles: Synthesis, Stability, and the Feaching Issue,” Appl. Organomet. Chem. 22, 288–299 (2008).
N. Miyaura and A. Suzuki, “Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds,” Chem. Rev. 95, 2457–2483 (1995).
N. Miyaura, “Cross-Coupling Reaction of Organoboron Compounds via Base-Assisted Transmetalation to Palladium(II) Complexes,” J. Organomet. Chem. 653, 54–57 (2002).
I. P. Beletskaya and A. V. Cheprakov, “Metal Complexes as Catalysts for C—C Cross-Coupling Reactions,” in Comprehensive Coordination Chemistry II: From Biology to Nanotechnology, Ed. by J. A. McCleverty and T. J. Meyer (Elsevier, Oxford, 2004), Vol. 9, pp. 305–368.
N. T. S. Phan, M. V. D. Sluys, and C. W. Jones, “On the Nature of the Active Species in Palladium Catalyzed Mizoroki—Heck and Suzuki—Miyaura Couplings—Homogeneous or Heterogeneous Catalysis: A Critical Review,” Adv. Synth. Catal. 348, 609–679 (2006).
V. P. Ananikov, D. G. Musaev, and K. Morokuma, “Theoretical Insight into the C—C Coupling Reactions of the Vinyl, Phenyl, Ethynyl, and Methyl Complexes of Palladium and Platinum,” Organometallics 24, 715–723 (2005).
V. P. Ananikov, D. G. Musaev, and K. Morokuma, “Critical Effect of Phosphane Ligands on the Mechanism of Carbon—Carbon Bond Formation Involving Palladium(II) Complexes: A Theoretical Investigation of Reductive Elimination from Square-Planar and T-Shaped Species,” Eur. J. Inorg. Chem., No. 34, 5390–5399 (2007).
V. P. Ananikov, D. G. Musaev, and K. Morokuma, “Transition-Metal Catalyzed Carbon—Carbon Bond Formation: The Key of Homogeneous Catalysis,” in Computational Modeling for Homogeneous and Enzymatic Catalysis: A Knowledge-Base for Designing Efficient Catalysts, Ed. by K. Morokuma and D. G. Musaev (Wiley, Weinheim, 2008), pp. 131–148.
A. A. C. Braga, G. Ujaque, and F. Maseras, “Mechanism of Palladium-Catalyzed Cross-Coupling Reactions,” in Computational Modeling for Homogeneous and Enzymatic Catalysis: A Knowledge-Base for Designing Efficient Catalysts, Ed. by K. Morokuma and D. G. Musaev (Wiley, Weinheim, 2008), pp. 109–130.
S. P. Nolan and O. Navarro, “C—C Bond Formation by Cross-Coupling,” in Comprehensive Organometallic Chemistry III: From Fundamentals to Applications, Ed. by D. M. P. Mingos and R. H. Crabtree (Elsevier, Oxford, 2007), Vol. 11, pp. 1–37.
I. P. Beletskaya, “The Cross-Coupling Reactions of Organic Halides with Organic Derivatives of Tin, Mercury, and Copper Catalyzed by Palladium,” J. Organomet. Chem. 250, 551–564 (1983).
I. P. Beletskaya, “Palladium Catalyzed C—C and C—Heteroatom Bond Formation Reactions,” Pure Appl. Chem. 69, 471–476 (1997).
R. Narayanan and M. A. El-Sayed, “Effect of Catalysis on the Stability of Metallic Nanoparticles: Suzuki Reaction Catalyzed by PVP-Palladium Nanoparticles,” J. Am. Chem. Soc. 125, 8340–8347 (2003).
Y Li, X. M. Hong, D. M. Collard, and M. A. El-Sayed, “Suzuki Cross-Coupling Reactions Catalyzed by Palladium Nanoparticles in Aqueous Solution,” Org. Lett. 2, 2385–2388 (2000).
Y Li, E. Boone, and M. A. El-Sayed, “Size Effects of PVP-Pd Nanoparticles on the Catalytic Suzuki Reactions in Aqueous Solution,” Langmuir 18, 4921–4925 (2002).
I. P. Beletskaya, A. N. Kashin, I. A. Khotina, and A. R. Khokhlov, “Efficient and Recyclable Catalyst of Palladium Nanoparticles Stabilized by Polymer Micelles Soluble in Water for Suzuki—Miyaura Reaction: Ostwald Ripening Process with Palladium Nanoparticles,” Synlett, No. 10, 1547–1552 (2008).
R. Narayanan and M. A. El-Sayed, “FTIR Study of the Mode of Binding of the Reactants on the Pd Nanoparticle Surface during the Catalysis of the Suzuki Reaction,” J. Phys. Chem. B 109, 4357–4360 (2005).
D. Astruc, “Palladium Nanoparticles as Efficient Green Homogeneous and Heterogeneous Carbon-Carbon Coupling Precatalysts: A Unifying View,” Inorg. Chem. 46, 1884–1894 (2007).
A. K. Diallo, C. Ornelas, L. Salmon, J. R. Aranzaes, and D. Astruc, “‘Homeopathic’ Catalytic Activity and Atom-Leaching Mechanism in Miyaura—Suzuki Reactions under Ambient Conditions with Precise Dendrimer-Stabilized Pd Nanoparticles,” Angew. Chem., Int. Ed. Engl. 46, 8644–8648 (2007).
R. Narayanan, C. Tabor, and M. A. El-Sayed, “Can the Observed Changes in the Size or Shape of a Colloidal Nanocatalyst Reveal the Nanocatalysis Mechanism Type: Homogeneous or Heterogeneous?” Top. Catal. 48, 60–74 (2008).
J. A. Widegren and R. G. Finke, “A Review of the Problem of Distinguishing True Homogeneous Catalysis from Soluble or Other Metal-Particle Heterogeneous Catalysis under Reducing Conditions,” J. Mol. Catal. A: Chem. 198, 317–341 (2003).
E. E. Finney and R. G. Finke, “Is It Homogeneous Pt(II) or Heterogeneous Pt(0)n Catalysis? Evidence That Pt(1.5-COD)C12 and Pt(1.5-COD)(CH3)2 Plus H2 from Heterogeneous, Nanoclusters Plus Bulk-Metal Pt(0) Hydrogenation Catalysts,” Inorg. Chim. Acta 359, 2979–2887 (2006).
I. P. Beletskaya and A. V. Cheprakov, “The Heck Reaction as a Sharpening Stone of Palladium Catalysis,” Chem. Rev. 100, 3009–3066 (2000).
N. J. Whitcombe, K. K. Hii, and S. E. Gibson, “Advances in the Heck Chemistry of Aryl Bromides and Chlorides,” Tetrahedron 57, 7449–7476 (2001).
J. G. de Vries, “The Heck Reaction in the Production of Fine Chemicals,” Can. J. Chem. 79, 1086–1092 (2001).
C. Amatore and A. Jutand, “Anionic Pd(0) and Pd(II) Intermediates in Palladium-Catalyzed Heck and Cross-Coupling Reactions,” Acc. Chem. Res. 33, 314–321 (2000).
V. P. Ananikov, D. G. Musaev, and K. Morokuma, “Catalytic Triple Bond Activation and Vinyl Plus Vinyl Reductive Coupling by Pt(IV) Complexes: A Density Functional Study,” Organometallics 20, 1652–1667 (2001).
V P. Ananikov, D. G. Musaev, and K. Morokuma, “Vinyl—Vinyl Coupling on Late Transition Metals through C—C Reductive Elimination Mechanism: A Computational Study,” J. Am. Chem. Soc. 124, 2839–2852 (2002).
F. Y Zhao, M. Shirai, Y Ikushima, and M. Arai, “The Leaching and Re-Deposition of Metal Species from and Onto Conventional Supported Palladium Catalysts in the Heck Reaction of Iodobenzene and Methyl Acrylate in N-Methylpyrrolidone,” J. Mol. Catal. A: Chem. 180, 211–219 (2002).
F. Zhao, K. Murakami, M. Shirai, and M. Arai, “Recyclable Homogeneous/Heterogeneous Catalytic Systems for the Heck Reaction through Reversible Transfer of Palladium Species between Solvent and Support,” J. Catal. 194, 479–483 (2000).
J. G. de Vries, “A Unifying Mechanism for All High-Temperature Heck Reactions: The Role of Palladium Colloids and Anionic Species,” Dalton Trans., No. 3, 421–429 (2006).
A. H. M. de Vries, J. M. C. A. Mulders, J. H. M. Mommers, H. J. W. Henderickx, and J. G. de Vries, “Homeopathic Ligand-Free Palladium as a Catalyst in the Heck Reaction: A Comparison with a Palladacycle,” Org. Lett. 5, 3285–3288 (2003).
A. H. M. de Vries, F J. Parlevliet, L. S. van de Vondervoort, J. H. M. Mommers, H. J. W. Henderickx, M. A. N. Walet, and J. G. de Vries, “A Practical Recycle of a Ligand-Free Palladium Catalyst for Heck Reactions,” Adv. Synth. Catal. 344, 996–1002 (2002).
M. B. Thathagar, J. E. Elshof, and G. Rothenberg, “Pd Nanoclusters in C—C Coupling Reactions: Proof of Leaching,” Angew. Chem., Int. Ed. Engl. 45, 2886–2890 (2006).
K. Köhler, R. G. Heidenreich, J. G. E. Krauter, and J. Pietsch, “Highly Active Palladium/Activated Carbon Catalysts for Heck Reactions: Correlation of Activity, Catalyst Properties, and Pd Leaching,” Chem. —Eur. J. 8, 622–631 (2002).
R. G. Heidenreich, E. G. E. Krauter, J. Pietsch, and K. Köhler, “Control of Pd Leaching in Heck Reactions of Bromoarenes Catalyzed by Pd Supported on Activated Carbon,” J. Mol. Catal. A: Chem. 182, 499–509 (2002).
I. P. Beletskaya and A. V. Cheprakov, “Palladacycles in Catalysis—A Critical Survey,” J. Organomet. Chem. 689, 4055–4082 (2004).
I. P. Beletskaya, A. N. Kashin, N. B. Karlstedt, A. V. Mitin, A. V. Cheprakov, and G. M. Kazankov, “NC-Palladacycles as Highly Effective Cheap Precursors for the Phosphine-Free Heck Reactions,” J. Organomet. Chem. 622, 89–96 (2001).
M. T. Reetz and G. Lohmer, “Propylene Carbonate Stabilized Nanostructured Palladium Clusters as Catalysts in Heck Reactions,” Chem. Commun. (Cambridge, UK), No. 16, 1921–1922 (1996).
M. T. Reetz and E. Westermann, “Phosphane-Free Palladium-Catalyzed Coupling Reactions: The Decisive Role of Pd Nanoparticles,” Angew. Chem., Int. Ed. Engl. 39, 165–168 (2000).
M. T. Reetz and J. G. de Vries, “Ligand-Free Heck Reactions Using Low Pd-Loading,” Chem. Commun. (Cambridge, UK), No. 14, 1559–1563 (2004).
C. Luo, Y Zhang, and Y Wang, “Palladium Nanoparticles in Poly(ethyleneglycol): The Efficient and Recyclable Catalyst for Heck Reaction,” J. Mol. Catal. A: Chem. 229, 7–12(2005).
H. Weingärtner, “Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies,” Angew. Chem., Int. Ed. Engl. 47, 654–670 (2008).
T. Welton, “Ionic Liquids in Catalysis,” Coord. Chem. Rev. 248, 2459–2477 (2004).
Z. C. Zhang, “Catalysis in Ionic Liquids,” Adv. Catal. 49, 153–237 (2006).
Y Zhou, “Recent Advances in Ionic Liquids for Synthesis of Inorganic Nanomaterials,” Curr. Nanosci. 1, 35–42 (2005).
T. Welton and P. J. Smith, “Palladium Catalyzed Reactions in Ionic Liquids,” Adv. Organomet. Chem. 51, 251–284(2004).
P. Migowski and J. Dupont, “Catalytic Applications of Metal Nanoparticles in Imidazolium Ionic Liquids,” Chem. Eur. J. 13, 32–39 (2007).
C. C. Cassol, A. P. Umpierre, G. Machado, S. I. Wolke and J. Dupont, “The Role of Pd Nanoparticles in Ionic Liquid in the Heck Reaction,” J. Am. Chem. Soc. 127, 3298–3299 (2005).
X. Yang, Z. Fei, D. Zhao, W H. Ang, Y Li, and P. J. Dyson, “Palladium Nanoparticles Stabilized by an Ionic Polymer and Ionic Liquid: A Versatile System for C—C Cross-Coupling Reactions,” Inorg. Chem. 47, 3292–3297 (2008).
D. Zhao, Z. Fei, T. J. Geldbach, R. Scopelliti, and P. J. Dyson, “Nitrile-Functionalized Pyridinium Ionic Liquids: Synthesis, Characterization, and Their Application in Carbon—Carbon Coupling Reactions,” J. Am. Chem. Soc. 126, 15 876–15 882 (2004).
W. K. Cho, J. K. Lee, S. M. Kang, Y S. Chi, H.-S. Lee, and I. S. Choi, “Gold-Catalyzed Cyanosilylation Reaction: Homogeneous and Heterogeneous Pathways,” Chem. —Eur. J. 13, 6351–6358 (2007).
A. T. Bell, “The Impact of Nanoscience on Heterogeneous Catalysis,” Science (Washington) 299, 1688–1691 (2003).
M. Haruta and M. Date, “Advances in the Catalysis of Au Nanoparticles,” Appl. Catal., A 222, 427–437 (2001).
C. Burda, X. Chen, R. Narayanan, and M. A. ElSayed, “Chemistry and Properties of Nanocrystals of Different Shapes,” Chem. Rev. 105, 1025–1102 (2005).
J. Le Bars, U. Specht, J. S. Bradley, and D. G. Blackmond, “A Catalytic Probe of the Surface of Colloidal Palladium Particles Using Heck Coupling Reactions,” Langmuir 15, 7621–7625 (1999).
R. Narayanan and M. A. El-Sayed, “Catalysis with Transition-Metal Nanoparticles in Colloidal Solution: Nanoparticle Shape Dependence and Stability,” J. Phys. Chem. B 109, 12 663–12 676 (2005).
M. DiVece, D. Grandjean, M. J.van Bael, C. P. Romero, X. Wang, S. Decoster, A. Vantomme, and P. Lievens, “Hydrogen-Induced Ostwald Ripening at Room Temperature in a Pd Nanocluster Film,” Phys. Rev. Lett. 100, 236 105 (4 pages) (2008).
R. S. Goeke and A. K. Datye, “Model Oxide Supports for Studies of Catalyst Sintering at Elevated Temperatures,” Top. Catal. 46, 3–9 (2007).
A. K. Datye, Q. Xu, K. C. Kharas, and J. M. McCarty “Particle Size Distributions in Heterogeneous Catalysts: What Do They Tell Us about the Sintering Mechanism?” Catal. Today 111, 59–67 (2006).
J. Hu and Y. B. Liu, “Pd Nanoparticle Aging and Its Implications in the Suzuki Cross-Coupling Reaction,” Langmuir 21, 2121–2123 (2005).
A. Howard, C. E. J. Mitchell, and R. G. Egdell, “Real Time STM Observation of Ostwald Ripening of Pd Nanoparticles on TiO2(110) at Elevated Temperature,” Surf. Sci. 515, L504–L508 (2002).
A. Imre, D. L. Beke, E. Gontier-Moya, I. A. Szabo, and E. Gillet, “Surface Ostwald Ripening of Pd Nanoparticles on the MgO(100) Surface,” Appl. Phys. A: Mater. Sci. Process. 71, 19–22(2000).
Y. Sun, B. Mayers, and Y. Xia, “Transformation of Silver Nanospheres into Nanobelts and Triangular Nanopiates through a Thermal Process,” Nano Lett. 3, 675–679 (2003).
K. M. Neyman, C. Inntam, V. A. Nasluzov, R. Kosarev, and N. Rösch, “Adsorption of d-Metal Atoms on the Regular MgO(00l) Surface: Density Functional Study of Cluster Models Embedded in an Elastic Polarizable Environment,” Appl. Phys. A: Mater. Sci. Proc. 78, 823–828 (2004).
C. Inntam, L. V. Moskaleva, K. M. Neyman, V. A. Nasluzov, and N. Rösch, “Adsorption of Dimers and Trimers of Cu, Ag, and Au on Regular Sites and Oxygen Vacancies of the MgO(00l) Surface: A Density Functional Study Using Embedded Cluster Models,” Appl. Phys. A: Mater. Sci. Proc. 82, 181–189 (2006).
C. Inntam, L. V. Moskaleva, I. V. Yudanov, K. M. Neyman, and N. Rösch, “Adsorption of Cu4, Ag4, and Au4 Particles on the Regular MgO(001) Surface: A Density Functional Study Using Embedded Cluster Models,” Chem. Phys. Lett. 417, 515–520 (2006).
V. Musolino, A. Selloni, and R. Car, “First-Principles Study of Adsorbed Cun (n = 1–4) Microclusters on MgO(100): Structural and Electronic Properties,” J. Chem. Phys. 108, 5044–5054 (1998).
K. M. Neyman, C. Inntam, L. V. Moskaleva, and N. Rösch, “Density Functional Embedded Cluster Study of Cu4, Ag4, and Au4 Species Interacting with Oxygen Vacancies on the MgO(00l) Surface,” Chem. Eur. J. 13, 277–286 (2007).
Metal Clusters at Surfaces, Ed. by K. H. Meiwes-Broer (Springer, Berlin, 2000).
M. Moseler, H. Häkkinen, and U. Landman, “Supported Magnetic Nanoclusters: Soft Landing of Pd Clusters on a MgO Surface,” Phys. Rev. Lett. 89, 176 103 (4 pages) (2002).
L. Giordano, C. Di Valentin, J. Goniakowski, and G. Pacchioni, “Nucleation of Pd Dimers at Defect Sites of the MgO(100) Surface,” Phys. Rev. Lett. 92, 096 105 (4 pages) (2004).
F Vies, F. Illas, and K. M. Neyman, “On the Mechanism of Formation of Metal Nanowires by Self-Assembly” Angew. Chem., Int. Ed. Engl. 46, 7094–7097 (2007).
I. P. Beletskaya and V P. Ananikov, “Unusual Influence of the Structures of Transition-Metal Complexes on Catalytic C—S and C—Se Bond Formation under Homogeneous and Heterogeneous Conditions,” Eur. J. Org. Chem. 3431–3444 (2007).
V P. Ananikov, N. V Orlov, I. P. Beletskaya, V. N. Khrustalev, M. Yu. Antipin, and T. V. Timofeeva, “New Approach for Size- and Shape-Controlled Preparation of Pd Nanoparticles with Organic Ligands: Synthesis and Application in Catalysis,” J. Am. Chem. Soc. 129, 7252–7253 (2007).
V. P. Ananikov, N. V. Orlov, and I. P. Beletskaya, “An Efficient and Convenient Synthesis of b-Vinyl Sulfides in Nickel Catalyzed Regioselective Addition of Thiols to Terminal Alkynes under Solvent-Free Conditions,” Organometallics 25, 1970–1977 (2006).
V. P. Ananikov, N. V. Orlov, and I. P. Beletskaya, “Highly Efficient Nickel-Based Heterogeneous Catalytic System with Nanosized Structural Organization for Selective Se—H Bond Addition to Terminal and Internal Alkynes,” Organometallics 26, 740–750 (2007).
V. P. Ananikov, S. S. Zalesskii, N. V. Orlov, and I. P. Beletskaya, “Nickel-Catalyzed Addition of Benzenethiol to Alkynes: Formation of Carbon—Sulfur and Carbon—Carbon Bonds,” Izv. Akad. Nauk, Ser. Khim., No. 11, 2030–2034 (2006) [Russ. Chem. Bull. 55 (11), 2109–2113 (2006)].
V. P. Ananikov, D. A. Malyshev, and I. P. Beletskaya, “NiCl2 Catalyzed Regioselective Hydrothiolation of Alkynes,” Adv. Synth. Catal. 347, 1993–2001 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.P. Ananikov, I.P. Beletskaya, 2010, published in Rossiiskie nanotekhnologii, 2010, Vol. 5, Nos. 1–2.
Rights and permissions
About this article
Cite this article
Ananikov, V.P., Beletskaya, I.P. Using nanosized, homogeneous, and heterogeneous catalytic systems in organic synthesis: changing the structure of active center in chemical reactions in solution. Nanotechnol Russia 5, 1–17 (2010). https://doi.org/10.1134/S1995078010010015
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078010010015