Abstract
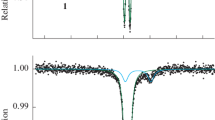
The spin state of the magnetic center for a spin-variable cation with nitrate [FeIII(3-OMe-Sal2trien)]NO3⋅H2O(I) and iodide [FeIII(3-OMe-Sal2trien)]I (II) anions is studied by Mössbauer spectroscopy on 57Fe nuclei (3-OMe-Sal2trien is the condensation product of triethylenetetramine with 3-methoxy salicylaldehyde) in the temperature ranges of 5 to 305 and 85 to 296 K, respectively. It was shown that, for both complexes, only one low-spin (S = 1/2) doublet from iron(III) ions appears in the spectra, despite significant differences (up to ~10%) in the length of the iron(III)–ligand bonds in the N4O2 coordination octahedron of the salt II compared with I, according to X-ray diffraction data at 293 K. The Debye temperatures were determined for both compounds, Θ = 157 and 153 K.






Similar content being viewed by others
REFERENCES
P. Gutlich and H. A. Goodwin, Top. Curr. Chem. 233, 1 (2004). https://doi.org/10.1007/b13527
V. Ya. Krivnov and D. V. Dmitriev, Russ. J. Phys. Chem. B 15, 89 (2021). https://doi.org/10.1134/S199079312101022X
M. V. Kirman and E. I. Kunitsyna, Russ. J. Phys. Chem. B 13, 408 (2019). https://doi.org/10.1134/S1990793119030187
A. V. Lobanov and M. Ya. Melnikov, Russ. J. Phys. Chem. B 13, 565 (2019). https://doi.org/10.1134/S1990793119040110
A. I. Kokorin, O. I. Gromov, T. Kálai, K. Hideg, and A. E. Putnikov, Russ. J. Phys. Chem. B 13, 739 (2019). https://doi.org/10.1134/S1990793119050178
V. Ya. Krivnov, D. V. Dmitriev, and N. S. Erikhman, Russ. J. Phys. Chem. B 13, 923 (2019). https://doi.org/10.1134/S1990793119060228
C.-M. Jureschi, J. Linares, A. Boulmaali, et al., Sensors 16, 187 (2016). https://doi.org/10.3390/s16020187
R. Pritchard, S. A. Barrett, C. A. Kilner, et al., Dalton Trans., 3159 (2008). https://doi.org/10.1039/B801892H
M. F. Tweedle and L. J. Wilson, J. Amer. Chem. Soc. 98, 4824 (1976). https://doi.org/10.1021/ja00432a023
Y. N. Shvachko, N. G. Spitsyna, D. V. Starichenko, et al., Molecules 25, 4922 (2020). https://doi.org/10.3390/molecules25214922
M. A. Blagov, V. B. Krapivin, S. V. Simonov, et al., Dalton Trans. 47, 16040 (2018). https://doi.org/10.1039/C8DT03619E
N. Spitsyna, N. Ovanesyan, M. Blagov, et al., Eur. J. Inorg. Chem. 48, 4556 (2020). https://doi.org/10.1002/ejic.202000873
Bruker TOPAS 5 User Manual (Bruker AXS GmbH, Germany, Karlsruhe, 2015).
M. Blume, Phys. Rev. 174 (2), 351 (1968). https://doi.org/10.1103/PhysRev.174.351
M. Blume, Phys. Rev. Lett. 18, 305 (1967). https://doi.org/10.1103/PhysRevLett.18.305
W. M. Reiff, Coord. Chem. Rev. 10, 37 (1973). https://doi.org/10.1016/S0010-8545(00)80231-3
S. R. Fletcher and T. C. Gibb, J. Chem. Soc., Dalton Trans., 309 (1977). https://doi.org/10.1039/DT9770000309
T. C. Gibb, J. Chem. Soc. A, 1439 (1968). https://doi.org/10.1039/J19680001439
S. Floquet, E. Riviére, K. Boukheddaden, et al., Polyhedron 80, 60 (2014). https://doi.org/10.1016/j.poly.2014.01.025
I. Nemec, R. Herchel, I. Sălitroă, et al., CrystEngComm., No. 14, 7015 (2012). https://doi.org/10.1039/C2CE25862E
Funding
This study was carried out as part of a state task of IPCP RAS (registration number АААА-А19-119092390079-8).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spitsyna, N.G., Ovanesyan, N.S. & Blagov, M.A. A Comparative Study of Nitrate and Iodide of the Spin-Variable Iron(III) Cation with the N4O2 Coordination Sphere by Mössbauer Spectroscopy. Russ. J. Phys. Chem. B 16, 565–571 (2022). https://doi.org/10.1134/S1990793122040157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122040157