Abstract
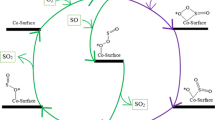
The Cr–carbon nanotubes and Cr–boron nitride nanotubesas novel catalysts were used to find out the details of mechanisms of CN oxidation. The oxidation of CN molecule can process via Cr–CNT and Cr–BNNT catalysts through the Langmuir–Hinshelwood (LH) and Eley–Rideal (ER) mechanisms. The CN molecule joins to Cr atom and Cr–surface–\({\text{O}}_{2}^{*}\) and Cr–surface–O* are created as important intermediate structures with low barrier energies. The cis-Cr–surface–OCNO* complex according to ER mechanisms more stable than four-elements–ring complex in LH mechanism, approximately 0.09 and 0.11 eV, respectively. In LH pathway, total activity was bounded by irretrievable adsorption of OCN molecules in Cr atom of Cr–CNT and Cr–BNNT. In ER pathway, two OCN molecules are released at normal temperature. The catalytic capabilities of Cr–CNT and Cr–BNNT to oxidation of CN molecule were demonstrated in this study.


Similar content being viewed by others
REFERENCES
Yu. N. Shebeko, V. V. Azatyan, I. A. Bolodyan, et al., Combust. Flame 121, 542 (2000).
A. Drakon and A. Eremin, Combust. Flame 162, 2746 (2015).
J. L. Pagliaro, G. T. Linteris, and P. T. Baker, Combust. Flame 162, 41 (2015).
V. I. Babushok, G. T. Linteris, and P. T. Baker, Combust. Flame 162, 1104 (2015).
G. Linteris, P. Sunderland, V. R. Katta, and O. Meier, Proc. Combust. Inst. 34, 2683 (2013).
Ya. A. Lisochkin and V. I. Poznyak, Combust. Explos. Shock Waves 37, 32 (2001).
V. I. Babushok, G. T. Linteris, and O. C. Meier, Combust. Flame 159, 3569 (2012).
V. S. Arutyunov, Russ. J. Gen. Chem. 54, 3 (2010).
V. S. Arutyunov, J. Nat. Gas Chem. 13, 10 (2004).
E. V. Sheverdenkin, and V. M. Rudakov, Theor. Found. Chem. Eng. 38, 311 (2004).
V. S. Arutyunov, V. I. Savchenko et al., Theor. Found. Chem. Eng. 39, 487 (2005).
V. I. Bykov, N. V. Kiselev, and S. B. Tsybenova, Dokl. Chem. 421, 161 (2008).
O. E. Leshakov and E. A. Mamash, Sib. Zh. Ind. Mat. 15 (3), 70 (2012).
O. E. Leshakov and M. P. Krasil’nikov, Sib. Zh. Ind. Mat. 16 (3), 116 (2013).
V. Ya. Basevich and S. M. Frolov, Russ. J. Phys. Chem. B 7, 161 (2013).
V. Ya. Basevich and S. M. Frolov, Russ. J. Phys. Chem. B 9, 268 (2015).
V. Ya. Basevich, S. N. Medvedev, and S. M. Frolov, Russ. J. Phys. Chem. B 9, 933 (2015).
S. Medvedev, V. S. Posvyanskii, and S. M. Frolov, Russ. J. Phys. Chem. B 10, 801 (2016).
S. S. Sergeev, S. M. Frolov, and B. Basara, Gorenie Vzryv 10 (2), 26 (2017).
S. M. Frolov, V. Ya. Basevich, and S. N. Medvedev, Dokl. Phys. Chem. 470, 150 (2016).
V. S. Posvyanskii, F. S. Frolov, and S. M. Frolov, Russ. J. Phys. Chem. B 4, 995 (2010).
V. Ya. Basevich and S. M. Frolov, Russ. J. Phys. Chem. B 4, 985 (2010).
V. Ya. Basevich, F. S. Frolov, B. Basara, and P. Prishing, Combust. Explos. Shock Waves 9, 36 (2016).
S. M. Frolov and F. S. Frolov, Russ. J. Phys. Chem. B 12, 245 (2018).
H. Machrafi and S. Cavadias, Combust. Flame 155, 557 (2008).
V. Ya. Basevich and S. M. Frolov, Russ. J. Phys. Chem. B 7, 161 (2013).
V. Ya. Basevich and S. M. Frolov, Russ. J. Phys. Chem. B 9, 268 (2015).
V. Ya. Basevich and S. M. Frolov, Russ. J. Phys. Chem. B 9, 933 (2015).
M. A. Oehlschlaeger and R. K. Hanson, Int. J. Chem. Kinet. 36, 67 (2004).
A. J. Cohen, P. Mori-Sánchez, and W. Yang, Chem. Rev. 112, 289 (2012).
E. G. Hohenstein, and S. T. Chill, J. Chem. Theory Comput. 4, 1996 (2008).
K. E. Riley, M. Pitoňák, P. Jurečka, and P. Hobza, Chem. Rev. 110, 5023 (2010).
L. Ferrighi, Y. Pan, H. Grönbeck, and B. Hammer, J. Phys. Chem. C 116, 7374 (2012).
N. Mardirossian and M. Head-Gordon, J. Chem. Theory Comput. 9, 4453 (2013).
L. Goerigk, J. Phys. Chem. Lett. 6, 3891 (2015).
N. Mardirossian and M. Head-Gordon, J. Chem. Theory Comput. 12, 4303 (2016).
E. DeCarlos and J. G. Ángyán, J. Chem. Phys. 145, 124105 (2016).
P. Kasper, J. Phys. Chem. A 121, 2022 (2017).
G. Michael and I. S. Bushmarinov, Science (Washington, DC, U. S.) 355, 49 (2017).
V. G. Ruiz, W. Liu, E. Zojer, and A. Tkatchenko, Phys. Rev. Lett. 108, 146103 (2012).
Y. Zhao and D. G. Truhlar, Theor. Chem. Acc. 120, 215 (2008).
Y. Huang, C. J. Sung, and J. A. Eng, Combust. Flame 139, 239 (2004).
K. Kumar, J. E. Freeh, C. J. Sung, and Y. Huang, J. Propuls. Power 23, 428 (2007).
V. Ya. Basevich, A. A. Belyaev, and S. M. Frolov, Russ. J. Phys. Chem. B 4, 995 (2010).
J. R. Yang, C. Y. Yukao, J. J. Whang, and S.-C. Wong, Combust. Flame 123, 266 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashraf, M.A., Liu, Z. & Najafi, M. DFT Study of CN Oxidation (CN + ½O2 → OCN) on the Surfaces of Chromium-Doped Nanotubes (Cr–CNT (8, 0) and Cr–BNNT (8, 0)). Russ. J. Phys. Chem. B 14, 217–221 (2020). https://doi.org/10.1134/S1990793120020189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793120020189