Abstract
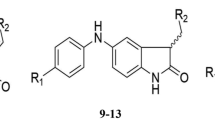
Quantitative structure-activity relationship (QSAR) of twenty-five different Etoposides derivatives was estimated by means of multiple linear regression (MLR), artificial neural network (ANN), simulated annealing (SA) and genetic algorithm (GA) techniques. The geometric compounds were selected as optimized samples using Gaussian 09W at B3LYP/6-31g. A high predictive ability was observed for the MLR-MLR, MLR-ANN, SA-ANN, MLR-GA and GA-ANN models, with the root mean sum square errors (RMSE) of 0.6265, 0.223, 0.195, 0.161 and 0.061 in gas phase and 0.5864, 0.226, 0.061, 0.106, and 0.0320 in the solvent phase, respectively (N = 25). The results obtained using the GA-ANN method indicated that the activity of derivatives of Etoposide depends on several parameters including Mor 14u, EEig12d, VEA1 and ICR descriptors in gas phase and RDF065p, Qxxe, ISH, RDF 050v and GATS6p descriptors in the solvent phase. Finally, the comparison of the quality of ANN with different MLR methods showed that ANN has a better predictive ability.
Similar content being viewed by others
References
Y. Pommier, E. Leo, H. Zhang, and C. Marchand, Chem. Biol. 17, 421 (2010).
K. R. Hande, Eur. J. Cancer 34, 15141 (1998).
M. Gordaliza, P. A. del Garcia, J. M. Corral, M. A. Castro, and M. A. Gómez-Zurita, Toxicon. 44, 441 (2004).
A. Nijnik et al., J. Clin. Invest. 119, 1696 (2009).
H. S. Lee, H. K. Yang, W.H. Kim, et al. Cancer Res. Treat. 37, 98 (2005).
S. Ekins and R. S. Obach, J. Pharmacol. Exp. Ther. 295, 463 (2000).
M. T. D. Cronin, Curr. Opin. Drug Discov. Dev. 3, 292 (2000).
F. Yoshida and J. G. Topliss, J. Med. Chem. 43, 2575 (2000).
R. Todeschini, QSAR Group. http://www.disat.unimib.it/chem.
A. R. Katritzky, V. S. Lobanov, and M. Karelson, Comprehensive Descriptors for Structural and Statistical Analysis, Reference Manual, Version 2.13 (1995).
A. R. Katritzky, V. Lobanov, and M. Karelson, Chem. Soc. Rev. 24, 279 (1995).
D. T. Manallack and D. J. Livingstone, Eur. J. Med. Chem. 34, 95 (1999).
D. Goldberg, Genetic Algorithms in Search, Optimization and Machine Learning (Addison-Wesley, Reading, MA, 1989).
H. X. Liu, R. S. Zhang, X. J. Yao, M. C. Liu, Z. D. Hu, and B. T. Fan, J. Chem. Inf. Comput. Sci. 43, 1288 (2003).
S. Putta, J. Eksterowicz, C. Lemmen, and R. Stanton, J. Chem. Inf. Comput. Sci. 43, 1623 (2003).
S. Gupta, M. Singh, and A. K. Madan, J. Chem. Inf. Comput. Sci. 39, 272 (1999).
V. Consonni, R. Todeschini, and M. Pavan, J. Chem. Inf. Comput. Sci. 42, 693 (2002).
E. B. DeMelo and M. M. Ferreira, Eur. J. Med. Chem. 44, 3577 (2009).
J. H. Schuur, P. Selzer, and J. Gasteiger, J. Chem. Inf. Comput. Sci. 36, 334 (1996).
R. Todeschini and V. Consonni, Handbook of Molecular Descriptors (Wiley-VCH, Weinheim, 2000).
M. C. Hemmer, V. Steinhauer, and J. Gasteiger, Vibrat. Spectrosc. 19, 151 (1999).
V. Consonni, R. Todeschini, and M. Pavan, J. Chem. Inf. Comput. Sci. 42, 682 (2002).
V. Consonni, R. Todeschini, and M. Pavan, J. Chem. Inf. Comput. Sci. 42, 693 (2002).
D. Horvath and B. Mao, QSAR. Comb. Sci. 22, 498 (2003).
P. Gramatica, V. Consonni, and R. Todeschini, Chemosphere 38, 1371 (1999).
P. Gramatica, V. Consonni, and R. Todeschini, Chemosphere 41, 763 (2000).
M. H. Fatemi and S. Gharaghani, Bioorg. Med. Chem. 15, 7746 (2007).
M. Jalali-Heravi and F. Parastar, J. Chem. Inf. Comput. Sci. 40, 147 (2000).
L. Kenneth, Quart. Appl. Math. 2, 164 (1944).
SPSS, Version 19 (2010). http://www.spssscience.com.
J. H. Schuur, P. Selzer, and J. Gasteiger, J. Chem. Inform. Comput. Sci. 36, 334 (1996).
T. Asadollahi, S. Dadfarnia, A. M. Haji Shabani, and J. B. Ghasemi, MATCH Commun. Math. Comput. Chem. 71, 287 (2014).
B. D. Silverman, J. Chem. Inform. Comput. Sci. 40, 1470 (2000).
S. H. Sadat Hayatshahi, P. Abdolmaleki, M. Ghiasi, and M. Safarian, FEBS Lett. 581, 506 (2007).
M. Nirouei, G. Ghasemi, P. Abdolmaleki, A. Tavakoli, and S. Shariati, Indian J. Biochem. Biophys. 49, 202 (2012).
www.pubchem.ncbi.nlm.nih.gov/Etoposide.Biological.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sayyadi kord Abadi, R., Alizadehdakhel, A. & Dorani Shiraz, S. Ab initio and QSAR study of several etoposides as anticancer drugs: Solvent effect. Russ. J. Phys. Chem. B 11, 307–317 (2017). https://doi.org/10.1134/S1990793117020130
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793117020130