Abstract
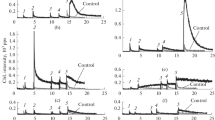
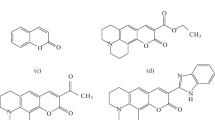
The cytochrome c–cardiolipin complex plays a key role in triggering apoptosis via the mitochondrial pathway due to lipoperoxidase and quasi-lipoxygenase activities of cytochrome c. As a result of the formation of this complex, the conformation of cytochrome c changes, which acquires the properties of peroxidase enzymes capable of triggering lipid peroxidation reactions. The functions of the cytochrome c–cardiolipin complex are usually studied using the recording of enhanced (activated) chemiluminescence. The chemiluminescence enhancer or activator increases the luminescence intensity due to the migration of the electron excitation energy from the excited lipid peroxidation products to the activator molecules, followed by its chemiluminescence with a high quantum yield. It is advisable to use in such studies activators that enhance luminescence without a chemical reaction with the components of the system under study and keep their concentration unchanged during the reaction time. At the end of the last century, it was shown on the Fe2+-induced lipid peroxidation system that quinolizidine derivatives of coumarin are such compounds. The ideas about the immutability of their concentration were transferred without additional studies to systems in which lipid peroxidation is triggered by peroxidase. However, it was found in this study by spectrophotometry using a reaction catalyzed by the cytochrome c–cardiolipin complex as an example that quinolizidine derivatives of coumarin, coumarin-314 (quinolizidine[5,6,7-gh]3-ethoxycarbonylcoumarin) and coumarin-334 (quinolizidine[5,6,7-gh]3-acetylcoumarin), are direct participants in the enzymatic lipoperoxidase reaction. Based on a comparison of changes in the concentration of coumarin derivatives in the presence and absence of phosphatidic acid, we found that coumarin derivatives are predominantly substrates of the second reaction of the peroxidase catalytic cycle, that is, the reduction of a peroxidase ferriform with two oxidized equivalents (compound 1) to a peroxidase ferriform with one oxidized equivalent (compound 2). It was also shown that during the catalysis of a quasi-hypoxygenase reaction (in the absence of H2O2 in the system), peroxidase passes through a catalytic cycle by the mechanism of one-electron oxidation followed by reduction, while there is no ferriform stage of peroxidase with two oxidized equivalents (compound 1). The rate constants of the first-order reaction of the decomposition of coumarin derivatives during the enzymatic lipoperoxidase reaction were determined, and based on them, functions were derived for calculating correction coefficients that take into account the decomposition of coumarin derivatives for correcting chemiluminograms obtained in the study of the cytochrome c–cardiolipin complex.




Similar content being viewed by others
REFERENCES
Kerr J.F., Wyllie A.H., Currie A.R. 1972. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 26 (4), 239–257. https://doi.org/10.1038/bjc.1972.33
Skulachev V.P. 1996. Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cell. FEBS Lett. 397 (1), 7–10.
Kalpage H.A., Bazylianska V., Recanati M.A., Fite A., Liu J., Wan J., Mantena N., Malek M.H., Podgorski I., Heath E.I., Vaishnav A., Edwards B.F., Grossman L.I., Sanderson T.H., Lee I., Huttemann M. 2019. Tissue-specific regulation of cytochrome c by post-translational modifications: Respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 33 (2), 1540–1553. https://doi.org/10.1096/fj.201801417R
Cain K., Bratton S.B., Langlais C., Walker G., Brown D.G., Sun X.M., Cohen G.M. 2000. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J. Biol. Chem. 275 (9), 6067–6070.
Shakeri R., Kheirollahi A., Davoodi J. 2017. Apaf-1: Regulation and function in cell death. Biochimie. 135, 111–125. https://doi.org/10.1016/j.biochi.2017.02.001
McComb S., Chan P.K., Guinot A., Hartmannsdottir H., Jenni S., Dobay M.P., Bourquin J.P., Bornhauser B.C. 2019. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 5 (7), eaau9433. https://doi.org/10.1126/sciadv.aau9433
Susin S.A., Daugas E., Ravagnan L., Samejima K., Zamzami N., Loeffler M., Costantini P., Ferri K.F., Irinopoulou T., Prevost M.C., Brothers G., Mak T.W., Penninger J., Earnshaw W.C., Kroemer G. 2000. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 192 (4), 571–580.
Bemani P., Mohammadi M., Hakakian A. 2018. Anti-ROR1 scFv-EndoG as a novel anti-cancer therapeutic drug. Asian Pac. J. Cancer Prev. 19 (1), 97–102. https://doi.org/10.22034/APJCP.2018.19.1.97
Li S., Wang T., Zhai L., Ge K., Zhao J., Cong W., Guo Y. 2018. Picroside II exerts a neuroprotective effect by inhibiting mPTP permeability and EndoG release after cerebral ischemia/reperfusion injury in rats. J. Mol. Neurosci. 64 (1), 144–155. https://doi.org/10.1007/s12031-017-1012-z
Kagan V.E., Tyurin V.A., Jiang J., Tyurina Y.Y., Ritov V.B., Amoscato A.A., Osipov A.N., Belikova N.A., Kapralov A.A., Kini V.V., Vlasova I.I., Zhao Q., Zou M., Di P., Svistunenko D.A., Kurnikov I.V., Borisenko G.G. 2005. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chem. Biol. 1, 223–232.
Kobayashi H., Nagao S., Hirota S. 2016. Characterization of the cytochrome c membrane-binding site using cardiolipin-containing bicelles with NMR. Angew. Chem. Int. Ed. Engl. 55 (45), 14019–14022. https://doi.org/10.1002/anie.201607419
Li M., Mandal A., Tyurin V.A., DeLucia M., Ahn J., Kagan V.E., P.C.A. van der Wel. 2019. Surface-binding to cardiolipin nanodomains triggers cytochrome c pro-apoptotic peroxidase activity via localized dynamics. Structure. 27 (5), 806–815. https://doi.org/10.1016/j.str.2019.02.007
Vladimirov Yu.A., Proskurnina E.V., Alekseev A.V. 2013. Molecular mechanisms of apoptosis. Structure of the cytochrome c–cardiolipin complex. Biochemistry (Moscow). 78 (10), 1086–1097.
Furtmuller P.G., Jantschko W., Zederbauer M., Jakopitsch C., Arnhold J., Obinger C. 2004. Kinetics of interconversion of redox intermediates of lactoperoxidase, eosinophil peroxidase and myeloperoxidase. Jpn. J. Infect. Dis. 57 (5), 830–831.
Rodriguez-Lopez J.N., Lowe D.J., Hernandez-Ruiz J., Hiner A.N., Garcia-Canovas F., Thorneley R.N. 2001. Mechanism of reaction of hydrogen peroxide with horseradish peroxidase: Identification of intermediates in the catalytic cycle. J. Amer. Chem. Soc. 123 (48), 11838–11847.
Nelson M.J., Seitz S.P. 1994. The structure and function of lipoxygenase. Curr. Opin. Struct. Biol. 4 (6), 878–884. https://doi.org/10.1016/0959-440x(94)90270-4
Chen G., Guo G., Zhou X., Chen H. 2020. Potential mechanism of ferroptosis in pancreatic cancer. Oncol. Lett. 19 (1), 579–587. https://doi.org/10.3892/ol.2019.11159
Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., Stockwell B.R. 2012. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 149 (5), 1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Zhang X., Xing X., Liu H., Feng J., Tian M., Chang S., Liu P., Zhang H. 2020. Ionizing radiation induces ferroptosis in granulocyte-macrophage hematopoietic progenitor cells of murine bone marrow. Int. J. Radiat. Biol. 96 (5), 584–595. https://doi.org/10.1080/09553002.2020.1708993
Demin E.M., Proskurnina E.V., Vladimirov Yu.A. 2008. Antioxidant action of dihydroquercetin and rutin in cytochrome c-catalyzed peroxidase reactions. Vestnik Moskovskogo Universiteta. Ser. 2. Khimia (Rus.). 49 (5), 354–360.
Russell G.A. 1957. Deuterium-isotope effects in the autoxidation of aralkyl hydrocarbons. Mechanism of the interaction of peroxy radicals. J. Amer. Chem. Soc. 79 (14), 3871–3877. https://doi.org/10.1021/ja01571a068
Belyakov V.A., Vassil’ev R.F. 1970. Chemiluminescence in hydrocarbon oxidation in solution. A quantitative study of the excitation and emission steps. Photochem. Photobiol. 11 (3), 179–192. https://doi.org/10.1111/j.1751-1097.1970.tb05986.x
Cilento G., Adam W. 1995. From free radicals to electronically excited species. Free Radic. Biol. Med. 19 (1), 103–114. https://doi.org/10.1016/0891-5849(95)00002-f
Vladimirov Yu.A., Sherstnev M.P., Azimbayev T.K. 1995. Coumarin-activated chemiluminescence of low-density lipoproteins in the presence of ferrous ions. Biofizika (Rus.). 40 (2), 323–327.
Vladimirov Yu.A., Sharov V.S., Driomina E.S., Reznitchenko A.V., Gashev S.B. 1995. Coumarin derivatives enhance the chemiluminescence accompanying lipid peroxidation. Free Radic. Biol. Med. 18 (4), 739–745.
Sharov V.S., Briviba K., Sies H. 1996. Assessment of the C-525 laser dye as a chemiluminescence sensitizer for lipid peroxidation in biological membranes: A comparison with chlorophyll-a. Free Radic. Biol. Med. 21 (6), 833–843.
Romodin L.A., Trifonova M.F., Lysenko N.P., Bekuzarova S.A. 2020. A method for determining the chemical participation of a chemiluminescence activator in a lipoperoxidase reaction. RF Patent No. 2 720 807. Application 04.06.2019, publ. 13.05.2020.
Romodin L.A., Vladimirov Yu.A., Lysenko N.P. 2019. Qualitative assessment of the interaction between various components of the reaction mixture used to study the lipoperoxidase activity of the cytochrome c complex with cardiolipin. Proceedings of the XI International Scientific and Practical Conference Transformation of experience of management of agribusiness of the European Union to Kazakhstan and Central Asia, Dulati readings-2019 (Rus.), p. 61–68.
Vladimirov Y.A., Proskurnina V.E. 2009. Free radicals and cellular chemiluminescence. Uspekhi biologicheskoy khimii (Rus.). 49, 341–388.
Vladimirov G.K. 2018. Structure and peroxidase function of cytochrome c complex with cardiolipin in an aqueous medium and in a non-polar environment. Diss. cand. sci. (Biol.). Moscow, Pirogov RNIMU of the Ministry of Health of the Russian Federation, 2018.
Zhuravlev A.I. 2009. Kvantovaya biofizika zhivotnih i cheloveka (Quantum biophysics of animals and humans). 3d ed. M.: MGAVMiB.
Zhuravlev A.I., Zubkova S.M. 2014. Antioksidanti. Svobodnoradikalnaya patologiya, starenie (Antioxidants. Free radical pathology, aging.) 2nd edition. Moscow: Belye Alva.
Romodin L.A., Vladimirov Yu.A., Shangin S.V., Vladimirov G.K., Lysenko N.P., Demikhov E.I. 2020. Isoquinoline coumarin derivatives as chemiluminescence activators in reactions of lipid peroxidation. Biophysics. 65 (4), 577–686. https://doi.org/10.1134/S0006350920040181
ACKNOWLEDGMENTS
The work was carried out using the funds of the basic budget financing and personal funds of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Puchkov
Abbreviations: C-314, coumarin-314, quinolizidine[5,6,7-gh]3-ethoxycarbonylcoumarin; C-334, coumarin-334, quinolizidine[5,6,7-gh]3-acetylcoumarin; CytC, cytochrome c; CytC–TOCL, cytochrome c complex with tetraoleoyl cardiolipin; J, chemiluminescence intensity; PA, dilinoleoyl phosphatidic acid; TOCL, tetraoleoyl cardiolipin; ε, molar absorption coefficient.
Rights and permissions
About this article
Cite this article
Romodin, L.A., Lysenko, N.P. & Pashovkin, T.N. The Use of Quinolizidine Derivatives of Coumarin in the Studies of the Mechanisms of Action of the Cytochrome c–Cardiolipin Complex. Biochem. Moscow Suppl. Ser. A 16, 158–166 (2022). https://doi.org/10.1134/S1990747822020064
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747822020064