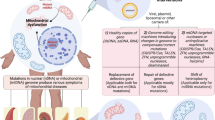
Abstract
Mitochondrial cytopathies are a heterogeneous group of systemic disorders caused by mutations in mitochondrial or nuclear genome. The review presents some data on pathogenic mutations in mitochondrial DNA leading to the imbalance in the oxidation phosphorylation processes and energy metabolism in the cells and eventually to the development of mitochondrial cytopathy. The pathways of medicated correction are examined, which are aimed at obtaining optimal energy efficiency of mitochondria with impaired functions, increase of the efficiency of energy metabolism in the tissues, as well as prevention of mitochondrial membrane damage by free radicals using antioxidants and membrane protectors. A conclusion is drawn on the inefficiency of currently used therapeutic strategies and the necessity of new approaches, which can be gene therapy of mitochondrial diseases. Some modern methods for gene defects correction, capable of restoring or removing the damaged gene, expressing full gene product, or blocking the mutant or strange genes work are analyzed. It is shown that the described approaches to the gene therapy of human mitochondrial diseases demand the introduction of foreign sequences into nuclear or mitochondrial genome of a living person, which completely excludes their practical application because of the uncertainty of the outcome. A perspective approach in solving this problem may be a creation of a system allowing the correction of defect genes without introducing synthetic nucleotides into the human genome. Phenotypic selection combined with a capacity of homologous recombination, artificially imparted to mitochondria of yeast Yarrowia lipolytica, allows for replication of intact human mitochondrial DNA in yeast mitochondria, supporting a full-size native human mitochondrial DNA in the yeast cells and eliminating pathogenic mutations by means of standard sitedirected PCR mutagenesis. After the correction in the Y. lipolytica cells, copies of mitochondrial DNA of an individual patient may be returned to him using the transfection of mesenchymal stromal cells followed by selection of transfectants grown in minimal culture media, in which the cells with higher respiratory mitochondrial activity will gain the advantage.
Similar content being viewed by others
References
Mazunin I.O., Volod’ko N.V., Starikovskaya E.B., Sukernik R.I. 2010. Human mitochondrial genome and mitochondrial diseases. Molekulyarnaya biologiya (Rus.). 44 (5), 755–772.
Harbauer A.B., Zahedi R.P., Sickmann A., Pfanner N., Meisinger C. 2014. The protein import machinery of mitochondria a regulatory hub in metabolism, stress, and disease. Cell Metab. 4, 357–372.
Spelbrink J.N. 2010. Functional organization of mammalian mitochondrial DNA in nucleoids: History, recent developments, and future challenges. IUBMB Life. 62, 19–32.
Nikitina L.P., Kuznetsova N.S., Gomboyeva A.Ts., Solovyeva N.V. 2011. Mitochondrial diseases, basic principles of their genetic classification (literature review), report II. Zabaykalskiy Med. Vestn. (Rus.). 2, 184–190.
Sverdlov E.D. 2009. Vzglyad na zhizn cherez okno genoma (The view of life through the genome window). Vol. 1. Moscow: Nauka. ISBN 978-5-02-034331-3, 978-5-02-034325-2.
Todorov I.N., Todorov G.I. 2009. Multifactor nature of high incidence of mtDNA mutations in mammalian somatic cells. Biochemistry (Moscow). 74, 971–979.
Patrushev M.V., Kamensky P.A., Mazunin I.O. 2014. Mitochondrial DNA mutations and methods of their correction. Biochemistry (Moscow). 79 (11), 1151–1161.
Lightowler R.N., Chinnery P.F., Turnbull D.M., Howell N. 1997. Mammalian mitochondrial genetics: Heredity, heteroplasmy and disease. Trends Genet. 13, 450–455.
Kmiec B., Woloszynska M., Janska H. 2006. Heteroplasmy as a common state of mitochondrial genetic information in plants and animals. Curr. Genet. 50, 149–159.
Cummins J.M. 2000. Fertilization and elimination of paternal mitochondrial genome. Hum. Reprod. 15, 92–101.
Hiraoka J., Hirao Y. 1988. Fate of sperm tail components after incorporation into the hamster egg. Gamete Res. 19, 369–380.
Zhao X., Li N., Guo W., Hu X., Liu Z., Gong G., Wang A., Feng J., Wu C. 2004. Further evidence for paternal inheritance of mitochondrial DNA in the sheep (Ovis aries). Heredity. 93, 399–403. doi 10.1038/sj.hdy.6800516
St. John J.C., Schatten G. 2004. Paternal mitochondrial DNA transmission during nonhuman primate nuclear transfer. Genetics. 167, 897–905.
Fontaine K.M., Cooley J.R., Simon C. 2007. Evidence for paternal leakage in hybrid periodical cicadas (Hemiptera: Magicicada spp.). PLoS One. 2, e892.
Dokianakis E., Ladoukakis E.D. 2014. Different degree of paternal mtDNA leakage between male and female progeny in interspecific Drosophila crosses. Ecol. Evol. 4, 2633–2641. doi 10.1002/ece3.106
Song W.H., Ballard J.W., Yi Y.J., Sutovsky P. 2014. Regulation of mitochondrial genome inheritance by autophagy and ubiquitin-proteasome system: Implications for health, fitness, and fertility. Biomed. Res. Int. 981867. doi 10.1155/2014/981867
Sutovsky P., McCauley T.C., Sutovsky M., Day B.N. 2003. Early degradation of paternal mitochondria in domestic pig (Sus scrofa) is prevented by selective proteasomal inhibitors lactacystin and MG132. Biol. Reprod. 68, 1793–1800.
Plotnikov E.Yu., Babenko V.A., Silachev D.N., Zorova L.D., Khryapenkova T.G., Savchenko E.S., Pevzner I.B., Zorov D.B. 2015. Intercellular transport of mitochondria. Biochemistry (Moscow). 80 (5), 542–548.
Wallace D.C., Lott M.T., Brown M.D., Kerstann K. 2001. Mitochondria and neuroophthalmologic diseases. In: The metabolic and molecular basis of inherited disease. Eds. Sctiver C.R., Beavdet A.L., Valle D.N.Y. VcGraw-Hill. 2, H2425–2512.
Swalwell H., Kirby D.M., Blakely E.L., Mitchell A., Salemi R., Sugiana C., Compton A.G., Tucker E.J., Ke B.X., Lamont P.J., Turnbull D.M., McFarland R., Taylor R.W., Thorburn D.R. 2011. Respiratory chain complex I deficiency caused by mitochondrial DNA mutations. Eur. J. Hum. Genet. 19 (7), 769–775.
Carelli V., La Morgia C., Valentino M.L., Barboni P., Ross-Cisneros F.N., Sadun A.A. 2009. Retinal gangli on cell neurodegeneration in mitochondrial inherited disorders. Biochim. Biophys. Acta. 1787, 518–528.
Yu-Wai-Man P., Griffiths P.G., Hudson G., Chinnery P.F. 2009. Inherited mitochondrial optic neuropathies. J. Med. Genet. 46, 145–158.
Rotig A. 2010. Genetic bases of mitochondrial respiratory chain disorders. Diabetes Metab. 36, 97–107.
A Human Mitochondrial Genome Database. 2009. www. mitomap.org
Finsterer J. 2007. Genetic, pathogenetic, and phenotypic implications of the mitochondrial A324. G tRNAL-eu(UUR) mutation. Acta Neurol. Scand. 116, 1–14.
Ugol'nik T.S., Manaenkova I.V. 2012. Nasledstvennye mitokhondrial’nye zabolevaniya (Hereditary mitochondrial diseases). Gomel State Medical University. ISBN 978-985-506-414-6.
Sukernik R.I., Derbeneva O.A., Starikovskaya E.B., Volod’ko N.V., Mikhaylovskaya I.E., Bychkov I.Yu., Lott M.T., Brown M.D., Wallace D.C. 2002. Human mitochondrial genome and mitochondrial diseases. Genetika (Rus.). 38 (2), 1–10.
Zorov D.B., Isaev N.K., Plotnikov E.Yu., Silachev D.N., Zorova L.D., Pevzner I.B., Morosanova M.A., Jankauskas S.S., Zorov S.D., Babenko V.A. 2013. Prospects of mitochondrial medicine. Biochemistry (Moscow). 78 (9), 979–990.
Joshi R., Strebel H.-P. 2003. Use of fumaric acid derivatives for treating mitochondrial diseases. US Patent 20030013761. A1.
Auguet M., De Lassauniere P.-E. C., Harnett J. 2006. Using thiazole derivatives for preparing medicinal agent designated for mitochondrion protection. Fr. Patent CA245563. A1.
von Borstel R. 2007. Compositions and methods for treatment of mitochondrial diseases. US Patent 20070010479. A1.
Skulachev V.P. 2008. A method of action on organism by the targeted delivery of biologically active substances into mitochondria, a pharmaceutical composition for its implementation, and a compound used for this purpose. RF Patent 02318500. C2.
Severina I.I., Severin F.F., Korshunova G.A., Sumbatyan N.V., Ilyasova T.M., Simonyan R.A., Rogov A.G., Trendeleva T.A., Zvyagilskaya R.A., Dugina V.B., Domnina L.V., Fetisova E.K., Lyamzaev K.G., Vyssokikh M.Y., Chernyak B.V., Skulachev M.V., Skulachev V.P., Sadovnichii V.A. 2013. In search of novel highly active mitochondria-targeted antioxidants: Thymoquinone and its cationic derivatives. FEBS Lett. 587, 2018–2024.
Skulachev V.P. 2013. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem. Biophys. Res. Commun. 441, 275–279.
Lukashev A.N., Skulachev M.V., Ostapenko V., Savchenko A.Y., Pavshintsev V.V., Skulachev V.P. 2014. Advances in development of rechargeable mitochondrial antioxidants. Prog. Mol. Biol. Transl. Sci. 127, 251–265.
Kim S.H., Kim H.J., Park H.S., Gu S.Y., Kwak H.S., Park D.H., Kim H.S., Cho H.J., Kim J.H., Kim J.Y., Park K.M. 2011. Pharmaceutical composition comprising indole compound. Patent WO2011052950. A3.
Delumeau J.-C., Martinet M., Reibaud M., Stutzmann J.-M. 1995. Application du riluzole dans le traitement des maladies mitochondriales. Fr. Patent WO1995019170. A1.
Bacman S.R., Williams S.L., Garcia S., Moraes C.T. 2010. Organ specific shifts in mtDNA heteroplasmy following systemic delivery of a mitochondria targeted restriction endonuclease. Gene Ther. 17, 713–720.
Doyle S.R., Chan C.K. 2009. Mitochondrial gene therapy: An evaluation of strategies for the treatment of mitochondrial DNA disorders. Hum. Gene Therapy. 19, 1355–1348.
Tonin Y., Heckei A.M., Dovydenko I., Meshaninova M., Comte C., Venyaminova A., Pyshnyi D., Tarassov I., Entelis N. 2014. Characterization of chemically modified oligonucleotides targeting a pathogenic mutation in human mitochondrial DNA. Biochimie. 100, 192–199.
Gammage P.A., Rorbach J., Vincent A.I., Rebar E.J., Minczuk M. 2014. Mitochondrial targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutation. EMBO Mol. Med. 6, 458–466.
Bacman S.R., Williams S.L., Pinto M., Peralta S., Moraes C.T. 2013. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 19 (9), 1111–1113.
Moraes C.T. 2014. A magic bullet to specifically eliminate mutated mitochondrial genomes from patients' cells. EMBO Mol. Med. 6 (4), 434–435.
Cermak T., Doyle E.L., Christian M., Wang L., Zhang Y., Voytas D.F. 2011. Efficient design and assembly of custom TALEN and other TAL effector based constructs for DNA targeting. Nucl. Acids Res. 39. e82.
Sanz A., Soilihi H., Portero-Otin M., Wilson A., Kemppainen E., McIlroy G., Ellilä S., Kemppainen K.K., Tuomela T., Lakanmaa M., Kiviranta E., Stefanatos R., Dufour E., Hutz B., Naudí A., Jové M., Zeb A., Vartiainen S., Matsuno-Yagi A., Yagi T., Rustin P., Pamplona R., Jacobs H.T. 2010. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc. Natl. Acad. Sci. USA. 107 (20), 9105–9110.
Marella M., Seo B.B., Thomas B.B., Matsuno-Yagi A., Yagi T. 2010. Successful amelioration of mitochondrial optic neuropathy using the yeast NDI1 gene in a rat animal model. PLoS One. 5, e11472.
Chadderton N., Arpad P., Millington-Ward S., Gobbo O., Overlack N., Carrigan M., O’Reilly M., Campbell M., Ehrhardt C., Wolfrum U., Humphries P., Kenna P.F., Farrar G.J. 2013. Intravitreal delivery of AAV-NDI1 provides functional benefit in a murine model of Leber hereditary optic neuropathy. Eur. J. Hum. Genet. 21 (1), 62–68.
Sander J.D., Joung J.K. 2014. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355. doi 10.1038/nbt.2842
Fogleman S., Santana C., Bishop C., Miller A., Capco D.G. 2016. CRISPR/Cas9 and mitochondrial gene replacement therapy: Promising techniques and ethical considerations. Am. J. Stem Cells. 5, 39–52.
Kolesnikova O., Kazakova H., Comte C., Steinberg S., Kamenski P., Martin R.P., Tarassov I., Entelis N. 2010. Selection of RNA aptamers imported into yeast and human mitochondria. RNA. 16 (5), 926–941.
Suzuki T., Nagao A., Suzuki T. 2011. Human mitochondrial tRNAs: Biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 45, 299–329.
Wang G., Shimada E., Zhang J., Hong J.S., Smith G.M., Teitell M.A., Koehler C.M. 2012. Correcting human mitochondrial mutations with targeted RNA import. Proc. Natl. Acad. Sci. USA. 109, 4840–4845.
Karicheva O.Z., Kolesnikova O.A., Schirtz T., Vysokikh M.Y., Mager-Heckel A.M., Lombes A., Boucheham A., Krasheninnikov I.A., Martin R.P., Entelis N., Tarassov I. 2011. Correction of the consequences of mitochondrial 324. A>G mutation in the MT-TL1 gene causing the MELAS syndrome by tRNA import into mitochondria. Nucl. Acids Res. 39, 8173–8186.
Comte C., Tonin Y., Heckel-Mager A.M., Boucheham A., Smirnov A., Auré K., Lombès A., Martin R.P., Entelis N., Tarassov I. 2013. Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with Kearns Sayre syndrome. Nucl. Acids Res. 41 (1), 418–433.
Yousif L.F., Stewart K.M., Kelley S.O. 2009. Targeting mitochondria with organelle specific compounds: Strategies and applications. ChemBioChem. 10 (12), 1939–1950.
Yousif L.F., Stewart K.M., Horton K.L., Kelley S.O. 2009. Mitochondria penetrating peptides: Sequence effects and model cargo transport. ChemBioChem. 10 (12), 2081–2088.
Boddapati S.V., D’Souza G.G., Weissig V. 2010. Liposomes for drug delivery to mitochondria. Methods Mol. Biol. 605, 295–303.
Michaud M., Ubrig E., Filleur S., Erhardt M., Ephritikhine G., Marechal-Drouardand L., Duchene A.M. 2014. Differential targeting of VDAC3 mRNA isoforms influences mitochondria morphology. Proc. Natl. Acad. Sci. USA. 111 (24), 8991–8996.
Wang G., Shimada E., Koehler C.M., Teitell M.A. 2012. PNPASE and RNA trafficking into mitochondria. Biochim. Biophys. Acta. 1819, 998–1007.
Sakhrani N.M., Padh H. 2013. Organelle targeting: Third level of drug targeting. Drug Des. Devel. Ther. 7, 585–599.
Biswas S., Torchilin V.P. 2014. Nanopreparations for organelle specific delivery in cancer. Adv. Drug Deliv. Rev. 66, 26–41.
Campbell C.T., Kolesar J.E., Kaufman B.A. 2012. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta. 1819 (9–10), 921–929.
Wang G., Chen H.W., Oktay Y., Zhang J., Allen E.L., Smith G.M., Fan K.C., Hong J.S., French S.W., McCaffery J.M., Lightowlers R.N., Morse H.C., Koehler C.M., Teitell M.A. 2010. PNPASE regulates RNA import into mitochondria. Cell. 142, 456–467.
Wang G., Shimada E., Zhang J., Hong J.S., Smith G.M., Teitell M.A., Koehler C.M. 2012. Correcting human mitochondrial mutations with targeted RNA import. Proc. Natl. Acad. Sci. USA. 109, 4840–4845. doi 10.1073/pnas.1116792109
Amato P., Tachibana M., Sparman M., Mitalipov S. 2014. Three-parent in vitro fertilization: Gene replacement for the prevention of inherited mitochondrial diseases. Fertil. Steril. 101, 31–35.
Tachibana M., Amato P., Sparman M., Woodward J., Sanchis D.M., Ma H., Gutierrez N.M., Tippner-Hedges R., Kang E., Lee H.S., Ramsey C., Masterson K., Battaglia D., Lee D., Wu D., Jensen J., Patton P., Gokhale S., Stouffer R., Mitalipov S. 2013. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 493, 627–631.
Cree L., Loi P. 2015. Mitochondrial replacement: From basic research to assisted reproductive technology portfolio tool—technicalities and possible risks. Mol. Hum. Reprod. 21, 3–10. doi 10.1093/molehr/gau082
Liu H., Wang C.W., Grifo J.A., Krey L.C., Zhang J. 1999. Reconstruction of mouse oocytes by germinal vesicle transfer: Maturity of host oocyte cytoplasm determines meiosis. Hum. Reprod. 14, 2357–2361.
Zhang J. 2015. Revisiting germinal vesicle transfer as a treatment for aneuploidy in infertile women with diminished ovarian reserve. J. Assist. Reprod. Genet. 32, 313–317.
Barritt J., Willadsen S., Brenner C., Cohen J. 2001. Cytoplasmic transfer in assisted reproduction. Hum. Reprod. Update. 7, 428–435.
Sanchis D.M., Ma H., Gutierrez N.M., Tippner-Hedges R., Kang E., Lee H.S., Ramsey C., Masterson K., Battaglia D., Lee D., Wu D., Jensen J., Patton P., Gokhale S., Stouffer R., Mitalipov S. 2013. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 493, 627–631.
Tachibana M., Amato P., Sparman M., Woodward J., Bredenoord A.L., Dondorp W., Pennings G., DeWert, G. 2011. Ethics of modifying the mitochondrial genome. J. Med. Ethics. 37, 97–100.
Tachibana M., Sparman M., Sritanaudomchai H., Ma H., Clepper L., Woodward J., Li Y., Ramsey C., Kolotushkina O., Mitalipov S. 2009. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 461, 367–372. doi 10.1038/nature08368
Gorman G.S., Grady J.P., Yi Ng, Schaefer A.M., McNally R.J., Chinnery P.F., Yu- Wai-Man P., Herbert M., Taylor R.W., McFarland R., Turnbull D.M. 2015. Mitochondrial donation — how many women could benefit? N. Engl. J. Med. 372, 885–887. doi 10.1056.NEJMc1500960
Hayakawa K., Esposito E., Wang X., Terasaki Y., Liu Y., Xing C., Ji X.,, Lo E.H. 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 535, 551–555. doi 10.1038/nature18928
Rustom A., Saffrich R., Markovic I., Walther P., Gerdes H.H. 2004. Nanotubular highways for intercellular organelle transport. Science. 303, 1007–1010.
Marzo L., Gousset K., Zurzolo C. 2012. Multifaceted roles of tunneling nanotubes in intercellular communication. Front Physiol. 3, 1–14.
Torralba D., Baixauli F., Sánchez-Madrid F. 2016. Mitochondria know no boundaries: Mechanisms and functions of intercellular mitochondrial transfer. Front. Cell Dev. Biol. 4, 107. doi 10.3389/fcell.2016.00107
Sinha P., Islam M.N., Bhattacharya S., Bhattacharya J. 2016. Intercellular mitochondrial transfer: Bioenergetic crosstalk between cells. Curr. Opin. Genet. Dev. 38, 97–101.
Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., Rehman R., Tiwari B.K., Jha K.A., Barhanpurkar A.P., Wani M.R., Roy S.S., Mabalirajan U., Ghosh B., Agrawal A. 2014. Miro1 regulates intercellular mitochondrial transport and enhances mesenchymal stem cell rescue efficacy. EMBO J. 33, 994–1010.
Spees J.L., Olson S.D., Whitney M.J., Prockop D.J. 2006. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA. Cell Biol. 103, 1283–1288. doi 10.1073/pnas.0510511103
Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. 2012. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759–765.
Li X., Zhang Y., Yeung S.C., Liang Y., Liang X., Ding Y., Ip M.S., Tse H.F., Mak J.C., Lian Q. 2014. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Resp. Cell Mol. Biol. 51, 455–446.
Ma H., Folmes C.D., Wu J., Morey R., Mora-Castilla S., Ocampo A., Ma L., Poulton J., Wang X., Ahmed R., Kang E., Lee Y., Hayama T., Li Y., Van Dyken C., Gutierrez N.M., Tippner-Hedges R., Koski A., Mitalipov N., Amato P., Wolf D.P., Huang T., Terzic A., Laurent L.C., Izpisua Belmonte J.C., Mitalipov S. 2015. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature. 524, 234–238. doi 10.1038/nature14546
Isakova E.P., Deryabina Yu.I., Belyakova A.V., Biryukova Yu.K., Teplova V.V., Shevelev A.B. 2016. The genetic system for human mitochondrial genome maintenance in the yeast Yarrowia lipolytica. Appl. Biochem. Microbiol. 52, 663–672.
Gonçalves F.A.G., Colen G., Takahashi J.A. 2013. Optimization of cultivation conditions for extracellular lipase production by Yarrowia lipolytica using response surface method. Afr. J. Biotechnol. 12, 2270–2278.
Liu J., Sneeden J., Heyer W.D. 2011. In vitro assays for DNA pairing and recombination-associated DNA synthesis. Methods Mol. Biol. 745, 363–383.
Isakova E.P., Deryabina Yu.I., Sekova V.Yu., Zyl’kova M.V., Kudykina Yu.K., Teplova V.V. 2015. Novel genetic system for protein transport across the mitochondrial membrane of Yarrowia lipolytica. Biol. Membrany (Rus.). 12, 346–351.
Isakova E.P., Epova E.Yu., Sekova V.Yu., Trubnikova E.V., Kudykina Yu.K., Zyl’kova M.V., Guseva M.A., Deryabina Yu.I. 2015. Construction of the Yarrowia lipolytica yeast strains capable of homologous recombination of mitochondrial genome. Appl. Biochem. Microbiol. 51, 336–341.
Isakova E.P., Deryabina Yu.I., Leonovich O.A., Zyl’kova M.V., Biryukova Yu.K. 2016. The study of accumulation of Bacillus subtilis protein RecA in the mitochondria of the recombinant Yarrowia lipolytica yeast strain. Appl. Biochem. Microbiol. 52, 174–183.
Nicaud J.M. 2012. Yarrowia lipolytica. Yeast. 29, 409–418.
Fritsch E.S., Chabbert C.D., Klaus B., Steinmetz L.M. 2014. A genome-wide map of mitochondrial DNA recombination in yeast. Genetics. 198, 755–771.
Babaev A.A. Ezhova G.P., Novikova N.A., Novikov V.V. 2007. Gennaya terapiya: korrektsiya geneticheskoi informatsii (Gene therapy: Correction of genetic information). Nizhny Novgorod: Lobachevsky State University of Nizhny Novgorod.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Teplova, Yu.I. Deryabina, E.P. Isakova, 2017, published in Biologicheskie Membrany, 2017, Vol. 34, No. 2, pp. 91–108.
Rights and permissions
About this article
Cite this article
Teplova, V.V., Deryabina, Y.I. & Isakova, E.P. Mitochondrial cytopathies: Their causes and correction pathways. Biochem. Moscow Suppl. Ser. A 11, 87–102 (2017). https://doi.org/10.1134/S1990747817020088
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747817020088