Abstract
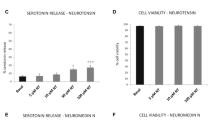
The present study focuses on possible ways to protect brain neurons during neuroinflammation. For the first time it is shown that peptide NPNDKYEPF amide, similar to activated protein C (APC), protects hippocampal neurons in a model of neuroinflammation induced by the toxic effects of endotoxin (lipopolysaccharide)-activated mast cells on neurons. It was found that the incubation of hippocampal neurons with mast cells activated by proinflammatory factors leads to neuronal apoptosis within 24 h after the exposure. Preincubation of mast cells with peptide NPNDKYEPF amide or with APC, before the toxin’s treatment, abolishes the toxic effects of the activated mast cells on neurons. By the blockade of protease-activated receptors of type 1 (PAR1), the receptor mechanism of the peptide action on mast cells and on neurons was identified. It was shown that PAR1 is required for the protective effect of the peptide in the conditions of neuroinflammation. Thus, peptide NPNDKYEPF amide is a neuroprotector, similar to APC, and can be used for the development of new approaches of the therapy of inflammatory processes accompanying different types of traumatic and ischemic brain damage.
Similar content being viewed by others
References
Mina Y., Rinkevich-Shop S., Konen E., Goitein O., Kushnir T., Epstein F.H., Feinberg M.S., Leor J., Landa-Rouben N. 2013. Mast cell inhibition attenuates myocardial damage, adverse remodeling, and dysfunction during fulminant myocarditis in the rat. J. Cardiovasc. Pharmacol. Ther. 18 (2), 152–161.
Zheng J., Wei C.C., Hase N., Shi K., Killingsworth C.R., Litovsky S.H., Powell P.C., Kobayashi T., Ferrario C.M., Rab A., Aban I., Collawn J.F., Dell’Italia L.J. 2014. Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog. PLoS One. 9 (4), e94732.
Strbian D., Kovanen P.T., Karjalainen-Lindsberg M.L., Tatlisumak T., Lindsberg P.J. 2009. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann. Med. 41 (6), 438–450.
Jin Y., Silverman A.J., Vannucci S.J. 2009. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 40 (9), 3107–3112.
Lindsberg P.J., Strbian D., Karjalainen-Lindsberg M.L. 2010. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J. Cereb. Blood Flow Metab. 30 (4), 689–702.
Nelissen S., Lemmens E., Geurts N., Kramer P., Maurer M., Hendriks J., Hendrix S. 2013. The role of mast cells in neuroinflammation. Acta Neuropathol. 125 (5), 637–650.
Biran V., Cochois V., Karroubi A., Arrang J.M., Charriaut-Marlangue C., Heron A. 2008. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 18 (1), 1–9.
Shibata M., Kumar S.R., Amar A., Fernandez J.A., Hofman F., Griffin J.H., Zlokovic B.V. 2001. Antiinflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 103 (13), 1799–1805.
Gorbacheva L., Pinelis V., Ishiwata S., Strukova S., Reiser G. 2010. Activated protein C prevents glutamate-and thrombin-induced activation of nuclear factor-κB in cultured hippocampal neurons. Neuroscience. 165, 1138–1146.
Wildhagen K.C., Schrijver R., Beckers L., ten Cate H., Reutelingsperger C.P., Lutgens E., Nicolaes G.A. 2014. Effects of exogenous recombinant APC in mouse models of ischemia reperfusion injury and of atherosclerosis. PLoS One. 9 (7), e101446.
Konanki R., Gulati S., Saxena R., Gupta A.K., Seith A., Kumar A., Saxena A., Kabra M., Kalra V., Lakshmy R. 2014. Profile of prothrombotic factors in Indian children with ischemic stroke. J. Clin. Neurosci. 21 (8), 1315–1318.
Berny-Lang M.A., Hurst S., Tucker E.I., Pelc L.A., Wang R.K., Hurn P.D., Di Cera E., McCarty O.J., Gruber A. 2011. Thrombin mutant W215A/E217A treatment improves neurological outcome and reduces cerebral infarct size in a mouse model of ischemic stroke. Stroke. 42 (6), 1736–1741.
Gorbacheva L.R., Storozhevykh T.P., Pinelis V.G., Davydova O., Ishiwata Sh., Strukova S.M. 2008. Activated protein C regulates neuronal survival at glutamate excitotoxicity via PAR1. Biokhimia (Rus.). 73 (6), 893–902.
Coughlin S.R. 2005. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3, 1800–1814.
Griffin J.H., Zlokovic B.V., Mosnier L.O. 2012. Protein C anticoagulant and cytoprotective pathways. Int. J. Hematol. 95, 333–345.
Shpacovitch V., Feld M., Hollenberg M.D., Luger T.A., Steinhoff M. 2008. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J. Leukoc. Biol. 83 (6), 1309–1322.
Gorbacheva L., Davidova O., Sokolova E., Ishiwata S., Pinelis V., Strukova S., Reiser G. 2009. Endothelial protein C receptor is expressed in rat cortical and hippocampal neurons and is necessary for protective effect of activated protein C at glutamate excitotoxicity. J. Neurochem. 111, 967–975.
Rusanova A.V., Vasilyeva T.V., Smirnov M.D., Strukova S. 2009. Mast cells as a target for anti-inflammatory action of APC. Tsitokiny i vospalenie (Rus.). 8 (3), 48–54.
Strukova S.M. 2001. Thrombin as a regulator of inflammation and reparative processes in tissues. Biochemistry (Mosc). 66 (1), 8–18.
Mosnier L.O., Sinha R.K., Burnier L., Bouwens E.A., Griffin J.H. 2012. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 120, 5237–5246.
Bouwens E.A., Stavenuiter F., Mosnier L.O. 2013. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J. Thromb. Haemost. 11 (1), 242–253.
Blank U., Madera-Salcedo I.K., Danelli L., Claver J., Tiwari N., Sanchez-Miranda E., Vazquez-Victorio G., Ramirez-Valadez K.A., Macias-Silva M., Gonzalez-Espinosa C. 2014. Vesicular trafficking and signaling for cytokine and chemokine secretion in mast cells. Front Immunol. 22 (5), 1–18.
da Silva E.Z., Jamur M.C., Oliver C. 2014. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 62 (10), 698–738.
Metcalfe D.D., Baram D., Mekori Y.A. 1997. Mast cells. Physiol. Rev. 77 (4), 1033–1079.
Schulke S., Flaczyk A., Vogel L., Gaudenzio N., Angers I., Loschner B., Wolfheimer S., Spreitzer I., Qureshi S., Tsai M., Galli S., Vieths S., Scheurer S. 2015. MPLA shows attenuated pro-inflammatory properties and diminished capacity to activate mast cells in comparison with LPS. Allergy. 70 (10), 1259–1268.
Brenner S.A., Zacheja S., Schaffer M., Feilhauer K., Bischoff S.C., Lorentz A. 2014. Soluble CD14 is essential for lipopolysaccharide-dependent activation of human intestinal mast cells from macroscopically normal as well as Crohn’s disease tissue. J. Immunology. 143 (2), 174–183.
Idriss H.T., Naismith J.H. 2000. TNF alpha and the TNF receptor superfamily: Structure-function relationship( s). Microsc. Res. Tech. 50 (3), 184–195.
Khodorov B.I., Storozhevykh T.P., Surin A.M., Sorokina E.G., Jurevicius A.I., Borodin A.V., Vinskya N.P., Khaspekov L.G., Pinelis V.G. 2001. Mitochondrial depolarization plays a dominant role in the mechanism of glutamate-induced disruption of neuronal calcium homeostasis. Biol. membrany (Rus.). 18 (6), 421–432.
Strbian D., Kovanen P.T., Karjalainen-Lindsberg M.L., Tatlisumak T., Lindsberg P.J. 2009. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann. Med. 41 (6), 438–450.
Gingrich M.B., Junge C.E., Lyuboslavsky P., Traynelis S.F. 2000. Potentiation of NMDA receptor function by the serine protease thrombin. J. Neurosci. 20 (12), 4582–4595.
Han K.S., Mannaioni G., Hamill C.E., Lee J., Junge C.E., Lee C.J., Traynelis S.F. 2011. Activation of protease activated receptor 1 increases the excitability of the dentate granule neurons of hippocampus. Mol. Brain. 10, 4–32.
Ide J., Aoki T., Ishivata S., Glusa E., Strukova S.M. 2007. Proteinase-activated receptor agonists stimulate the increase in intracellular Ca2+ in cardiomyocytes and proliferation of cardiac fibroblasts from chick embryos. Bull. Exp. Biol. Med. 144 (6), 760–763.
Mao Y., Jin J., Kunapuli S.P. 2008. Characterization of a new peptide agonist of the protease-activated receptor-1. Biochem. Pharmacol. 75 (2), 438–447.
He Q., Bao L., Zimering J., Zan K., Zhang Z., Shi H., Zu J., Yang X., Hua F., Ye X., Cui G. 2015. The protective role of (–)-epigallocatechin-3-gallate in thrombininduced neuronal cell apoptosis and JNK-MAPK activation. Neuroreport. 26, 416–423.
Zundorf G., Reiser G. 2011. The phosphorylation status of extracellular-regulated kinase 1/2 in astrocytes and neurons from rat hippocampus determines the thrombin-induced calcium release and ROS generation. J. Neurochem. 119, 1194–1204.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.I. Babkina, S.M. Strukova, V.G. Pinelis, G. Reiser, L.R. Gorbacheva, 2016, published in Biologicheskie Membrany, 2016, Vol. 33, No. 1, pp. 70–79.
Rights and permissions
About this article
Cite this article
Babkina, I.I., Strukova, S.M., Pinelis, V.G. et al. New synthetic peptide protects neurons from death induced by toxic influence of activated mast cells via protease-activated receptor. Biochem. Moscow Suppl. Ser. A 10, 126–134 (2016). https://doi.org/10.1134/S1990747816010037
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747816010037