Abstract
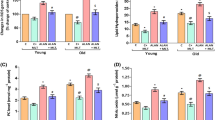
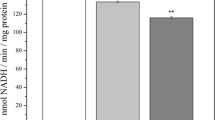
Oxidative stress is the major factor affecting different organs during aging. Mitochondria are considered to be a source of endogenous oxidants, concentrations of which can be maintained at a low level by antioxidant enzymes. At present, the aging process is considered to be tightly related with mitochondrial dysfunction, one of the reasons of which might be an increased sensitivity to induction of permeability transition pore in the inner membrane of mitochondria. Currently, the role of melatonin, concentration of which is lowered with aging, is widely examined. In the present study, the effect of melatonin on characteristics of stress-induced mPTP opening in mitochondria isolated from young and old rats, treated or not treated with melatonin, has been examined. Oxidative stress was induced by 1 or 100 μM cumene hydroperoxide. It was found that chronic treatment of old rats with melatonin caused suppression of mPTP opening in mitochondria. Melatonin was found to be able to prevent the cumene hydroperoxide-induced swelling of mitochondria isolated from melatonin-treated rats.
Similar content being viewed by others
References
Guevera R., Gianotti M., Roca P., Oliver J. 2011. Age and sex-related changes in rat brain mitochondrial function. Cell Physiol. Biochem. 27, 201–206.
Long J., Gao F., Tong L., Cotman C.W., Ames B.N., Liu J. 2009. Mitochondrial decay in the brains of old rats: Ameliorating effect of α-lipoic acid and acetyl-L-carnitine. Neurochem. Res. 34 (4), 755–763.
Harman D. 1956. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300.
Harman D. 1972. The biological clock: The mitochondria. J. Am. Geriatr. Soc. 20, 99–117.
Miquel J., Economos A.C., Fleming J., Johnson J.E. 1980. Mitochondrial role in cell aging. Exp. Gerontol. 15 (6), 575–591.
Tian L., Cai Q., Wei H. 1998. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med. 24, 1477–1484.
Gemma C., Vila J., Bachstetter A., Bickford P.C. 2007. Oxidative stress and the aging brain: From theory to prevention. In: Brain aging: Models, methods, and mechanisms. Riddle D.R., ed. CRC Press, Boca Raton (FL). Chapter 15.
Szalai G., Krishnamurthy R., Hajnyczky G. 1999. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 18 (22), 6349–6361.
Halestrap A.P., McStay G.P., Clarke S.J. 2000. The permeability transition pore complex: Another view. Biochimie. 84 (2–3), 153–166.
Bernardi P. 1999. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79 (4), 1127–1155.
Krestinina O.V., Odinokova I.V., Baburina Yu.L., Azarashvili T.S. 2013. Age-related effect of melatonin on permeability transition pore opening in rat brain mitochondria. Biochem. (Moscow) Suppl. Series A: Membr. Cell Biol. 7 (4), 286–293.
Falcon J., Besseau L., Fuentes M., Sauzet S., Magnanou E., Boeuf G. 2009. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann. N. Y. Acad. Sci. 1163, 101–111.
Reiter R.J. 1994. Pineal function during aging: attenuation of the melatonin rhythm and its neurobiological consequences. Acta Neurobiol. Exp. (Wars.). 54 (Suppl), 31–39.
Tan D.X., Manchester L.C., Hardeland R., Lopez-Burillo S., Mayo J.C., Sainz R.M., Reiter R.J. 2003. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant. J. Pineal Res. 34, 75–78.
Tan D.X., Chen L.D., Poeggeler B. 1993. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1, 57–60.
Menendez-Pelaez A., Reiter R.J. 1993. Distribution of melatonin in mammalian tissues: The relative importance of nuclear versus cytosolic localization. J. Pineal Res. 15 (2), 59–69.
Acuna-Castroviejo D., Escames G., Leon J., Carazo A., Khaldy H. 2003 Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 527, 549–557.
Lopez A., Garcia J.A., Escames G., Venegas C., Ortiz F., Lopez L.C., Acuna-Castroviejo D. 2009. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 46, 188–198.
Karasek M. 2004. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 39, 1723–1729.
Anisimov V.N. 2008. Molecularnie i fiziologicheskie mehanizmi stareniya (Molecular and physiological mechanisms of aging). Nauka, St. Petersburg. Vol. 2.
Acuna-Castroviejo D., Martin M., Macias M., Escames G., Leon J., Khaldy H., Reiter R.J. 2001. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 30 (2), 65–74.
Barja de Q.G., Perez-Campo R., Lopez T.M. 1990. Anti-oxidant defenses and peroxidation in liver and brain of aged rats. Biochem. J. 272, 247–250.
Dogru-Abbasoglu S., Ugurnal B., Tamer-Toptani S., Akdeniz S., Aykac-Toker G., Kocak-Toker N., Uysal M. 1998. Mitochondrial lipid peroxidation and antioxidant system in aged rats. Arch. Gerontol. Geriatr. 27, 35–40.
Rao G., Xia E., Richardson A. 1990. Effect of age on the expression of antioxidant enzymes in male Fischer F344 rats. Mech. Ageing Dev. 53, 49–60.
Vanella A., Villa R.F., Gorini A., Campisi A., Giuffrida-Stella A.M. 1989. Superoxide dismutase and cytochrome oxidase activities in light and heavy synaptic mitochondria from rat cerebral cortex during aging. J. Neurosci. Res. 22, 351–355.
Carrillo M.C., Kanai S., Sato Y., Kitani K. 1992. Agerelated changes in antioxidant enzyme activities are region and organ, as well as sex, selective in the rat. Mech. Ageing Dev. 65, 187–198.
Danh H.C., Benedetti M.S., Dostert P. 1983. Differential changes in superoxide dismutase activity in brain and liver of old rats and mice. J. Neurochem. 40, 1003–1007.
Öztürk G., Akbulut K.G., Güney Ş., Acuna-Castroviejo D. 2012. Age-related changes in the rat brain mitochondrial antioxidative enzyme ratios: Modulation by melatonin. Exp. Gerontol. 47 (9), 706–711.
Petrosillio G., Moro N., Paradies V., Ruggiero F.M., Paradies G. 2010. Increased susceptibility to Ca2+-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: Effect of melatonin. J. Pineal. Res. 48 (4), 340–346.
Sims N.R. 1990. Rapid isolation of metabolically active mitochondria from rat brain and subregions using percoll density gradient centrifugation. J. Neurochem. 55, 698–707.
Azarashvili T.S., Grachev D.E., Krestinina O.V., Evtodienko Yu.V., Yurkov I.S., Papadopoulos V., Reizer G. 2007. The peripheral-type benzodiazepine receptor is involved in control of Ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium. 42 (1), 27–39.
Slominski A., Tobin D.J., Zmijewski M.A., Wortsman J., Paus R. 2008. Melatonin in the skin: Synthesis, metabolism, and functions. Trends Endocrinol. Metab. 19, 17–24.
Bokov A., Chaudhuri A., Richardson A. 2004. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 125 (10–11), 811–826.
Gonca A.K., Gonul B., Akbulut H. 1999. Differential effects of pharmacological doses of melatonin on malondialdehyde and glutathione levels in young and old rats. Gerontology. 45, 67–71.
Kumar P., Taha A., Kale R.K., Cowsik S.M., Baquer N.Z. 2011. Physiological and biochemical effects of 17β-estradiol in aging female rat brain. Exp. Gerontol. 46, 597–605.
Krestinina O.V., Kruglov A.G., Grachev D.E., Baburina Yu.L., Evtodienko Yu.V., Moshkov D.A., Santalova I.M., Azarashvili T.S. 2010. Age-dependent changes of mitochondrial functions in Ca2+-induced opening of permeability transition pore. Biochem. (Moscow) Suppl. Series A: Membr. Cell Biol. 4 (2), 180–186.
Mather M., Rottenberg H. 2000. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem. Biophys. Res. Commun. 273, 603–608.
Petrosillo G., Casanova G., Matera M., Ruggiero F.M., Paradies G. 2006. Interaction of peroxidized cardiolipin wit rat heart mitochondrial membranes: Induction of permeability transition and cytochrome c release. FEBS Lett. 580, 6311–6316.
Pieri C. 1994. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 15, PL271–PL276.
Martin M., Macias M., Leon J., Escames G., Khalde H., Acuna-Castroviejo D. 2002. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Intern. J. Biochem. 34, 348–357.
Antolin I., Rodriguez C., Sainz R.M., Mayo J.C., Uria H., Kotler M.N., Rodriguez-Colunga M.J., Tolivia D., Menendez-Pelaez A. 1996. Neurohormobe melatonun prevents cell damage: Effect on gene expression for antioxidant enzymes. FASEB J. 10, 882–890.
Eckert G.P., Schiborr C., Hagl S., Abdel-Kader R., Muller W.E., Rimbach G., Frank J. 2013. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 62 (5), 595–602.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Krestinina, Yu.L. Baburina, T.S. Azarashvili, 2014, published in Biologicheskie Membrany, 2014, Vol. 31, No. 2, pp. 95–103.
Rights and permissions
About this article
Cite this article
Krestinina, O.V., Baburina, Y.L. & Azarashvili, T.S. Effect of melatonin on stress-induced opening of non-selective pore in mitochondria from brain of young and old rats. Biochem. Moscow Suppl. Ser. A 9, 116–123 (2015). https://doi.org/10.1134/S1990747814020032
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747814020032