Abstract
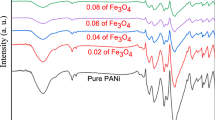
Poly(2-ethylaniline) (PEAN), poly(2-ethylaniline)-nanocomposite-Fe2O3 (PEAN/Fe2O3) and poly(2-ethylaniline)-nanocomposite-SiO2 (PEAN/SiO2) are synthesized by emulsion polymerization and characterized by UV–Visible, FTIR spectroscopy, Powder XRD, TGA, DTA, and SEM-EDX. The nanocomposites are amorphous and exhibit a three-step thermal degradation corresponding to the loss of moisture, loss of dopant, and the decomposition of the polymer composites. The electrical conductivity of the semi-conducting emeraldine salt forms of PEAN, PEAN/Fe2O3 and PEAN/SiO2 doped with chloride ions and camphor sulphonate ions are 4.3 × 10–4, 3.6 × 10–5, and 4.8 × 10–3 S/cm respectively. PEAN, PEAN/Fe2O3 and PEAN/SiO2 show excellent antibacterial activity against the gram-positive bacteria Staphylococcus aureus, moderate activity against Salmonella typhi and Klebsiella pneumoniae, and weak activity against Bacillus subtilis and Enterococcus faecalis. These materials are inactive against Escherichia coli. The destruction of the bacterial cell membranes due to the stronger interaction between the doped polycation chains and the negatively charged bacterial cell wall, and the release of Fe3+ ions due to electrostatic interaction facilitates the binding to the negatively charged bacterial cell membrane. The large surface area and the high content of SiOH groups in nanoporous silica facilitates the attachment with the surface of the bacterial cell walls. The nanocomposites demonstrate relatively good free radical scavenging activity at a concentration of 50 µL.











Similar content being viewed by others
REFERENCES
S. Jadoun, U. Riaz, and V. Budhiraja, Med. Devices Sens. 4 (1), e10141 (2020).
R. M. Sheltami, I. Abdullah, I. Ahmad, A. Dufresne, and H. Kargarzadeh, Carbohydr. Polym. 88, 772 (2012).
R. Balint, N. J. Cassidy and S. H. Cartmell, Acta Biomater. 10, 2341 (2014).
H. Nam, T. An, and G. Lim, Nanoscale Res. Lett. 9, 566 (2014).
J. P. Saikia, S. Banerjee, B. K. Konwar, and A. Kumar, Colloids Surf., B 81, 158 (2010).
M. R. Gizdavic-Nikolaidis, J. R. Bennett, S. Swift, A. J. Easteal, and M. Ambrose, Acta Biomater. 7, 4204 (2011).
S. Sharma and D. Kumar, Indian J. Eng. Mater. Sci. 17, 231 (2010).
N. L. Shi, X. M. Guo, H. M. Jing, J. Gong, C. Sun, and K. Yang, J. Mater. Sci. Technol. 22, 289 (2006).
M. G. Nikolaidis, J. T Sejdic, G. A. Bowmaker, R. P. Cooney, C. Thompson, and P. A. Kilmartin, Curr. Appl. Phys. 4, 347 (2004).
C. F. Hsu, H. Peng, C. Basle, J. Travas-Sejdic, and P. A. Kilmartin, Polym. Int. 60, 69 (2011).
J. H. Holtz and S. A. Asher, Nature 389 (6653), 829 (1997).
I. Willner, B. Willner, and E. Katz, Bioelectrochemistry 70, 2 (2007).
E. Parthiban, N. Kalaivasan, and S. Sudarsan, Arabian J. Chem. 13, 4751 (2020).
M. Kooti, P. Kharazi, and H. Motamedi, J. Taiwan Inst. Chem. Eng. 45, 2698 (2014).
S. Kant, S. Kalia, and A. Kumar, J. Alloys Compd. 578, 249 (2013).
S. Sultana, Rafiuddin, M. Z. Khan, K. Umar, and M. Muneer, J. Mater. Sci. Technol. 29, 795 (2013).
C. Sridhar, N. G. Yernale, and M. V. N. Ambika Prasad, Int. J. Chem. Eng. 2016, Article ID 3479248 (2016).
X. Quan, J. Wang, T. Souleyman, W. Cai, S. Zhao, and Z. Wang, Prog. Org. Coat. 124, 61 (2018).
M. N. Ahmad, M. N. Anjum, F. Nawaz, S. Iqbal, M. J. Saif, T. Hussain, A. Mujahid, M. U. Farooq, M. Nadeem, A. Rahman, A. Raza, and K. Shehzad, Polym. Compos. 39, 4524 (2017).
W. Cai, J. Wang, X. Quan, and Z. Wang, J. Appl. Polym. Sci. 135, 45657 (2018).
A. S. Al-Hussaini and W. Eldars, Des. Monomers Polym. 17, 458 (2014).
J. Wang, L. H. Zhu, J. Li, and H. Q. Tang, Chin. Chem. Lett. 18, 1005 (2007).
P. S. Jyoti, B. Somik, K. K. Bolin, and K. Ashok, Colloids Surf., B 81, 158 (2010).
E. N. Zare and M. M. Lakouraj, Iran. Polym. J. 23, 257 (2014).
A. L. Schemid, S. I. C. Torresi, A. N. Bassetto, and I. A. Carlos, J. Braz. Chem. Soc. 11, 317 (2000).
P. I. P Soares, D. Machado, C. Laia, L. C. J Pereira, J. T. Coutinho, I. M. M. Ferreira, C. M. M. Novo, and J. P. Borges, Carbohydr. Polym. 149, 382 (2016).
W. Qin, F. Vautard, P. Askeland, J. Yu, and L. T. Drzal, Polym. Compos. 38, 1474 (2015).
P. Boomi, H. G. Prabu, and J. Mathiyarasu, Colloids Surf., B 103, 9 (2013).
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C.R. Evans, Free Radicals Biol. Med. 26, 1231 (1999).
S. Bilal, S. Gul, R. Holze, and A.H. Ali Shah, Synth. Met. 206, 131 (2015).
M. G. Roe, J. M. Ginder, P. E. Wigeon, A. J. Epstein, M. Angelopoulos, and A. G. MacDiarmid, Phys. Rev. Lett. 60, 2789 (1999).
A. Gruger, A. Novak, A. Regis, and P. Columban, J. Mol. Struct. 328, 153 (1994).
J. Tang, X. Jing, B. Wang, and F. Wang, Synth. Met. 24, 231 (1988).
A. M. Mazrouaa, M. G. Mohamed, and M. Fekry, Egypt. J. Pet. 28, 165 (2019).
J. R. Martinez, F. Ruiz, Y. V. Vorobiev, F. P. Robles, and J.G. Hernandez, J. Chem. Phys. 109, 7511 (1998).
R. F. S. Lenza and W. L. Vasconcelos, Mater. Res. 5, 497 (2002).
D. Anakli and S. Cetinkaya, Curr. Appl. Phys. 10, 401 (2010).
P. Linganathan, J. Sundararajan, J. M. Samuel, J. Compos. 2014, Article ID 838975 (2014).
N. M. Chola, S. Sreenath, B. Dave, and R. K. Nagarale, Electrophoresis 40, 2979 (2019).
N. P. S. Chauhan, R. Ameta, R. Ameta, and S. C. Ameta, J. Indian Counc. Chem. 27, 128 (2010).
P. K. Prabhakar, S. Raj, P. R. Anuradha, S. N. Sawant, and M. Doble, Colloids Surf., B 86, 146 (2011).
X. Liang, M. Sun, L. Li, R. Qiao, K. Chen, Q. Xiao, and F. Xu, Dalton Trans. 41, 2804 (2012).
A. N. Andriianova, L. R. Latypova, L. Y. Vasilova, S. V. Kiseleva, V. V. Zorin, I. B. Abdrakhmanov, and A. G. Mustaffin, J. Appl. Polym. Sci. 138, 51397 (2021).
M. S. Alshammari, A. A. Essawy, A. M. El-Nggar, and S. M. Sayyah, J. Chem. 2020, Article ID 3297184 (2020).
V. L. Prasanna and R. Vijayaraghavan, Langmuir 31, 9155 (2015).
V. Stanic and S. B. Tanaskovic, in Nanotoxicity. Prevention and Antibacterial Applications of Nanomaterials, Ed. By S. Rajendran, A. Mukherjee, T. A. Nguyen, C. Godugu, and R. K. Shukla (Elsevier, Amsterdam, 2020), Chap. 11, pp. 241–274.
K. Pandiselvi and S. Thambidurai, Mater. Sci. Semicond. Process. 31, 573 (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Julia Sebastian, Jhancy Mary, S. Structural, Thermal and Electrochemical Behavior of Poly(2-ethylaniline)-nanocomposite-Fe2O3 and Poly(2-ethylaniline)-nanocomposite-SiO2 for Antibacterial and Antioxidant Studies. Polym. Sci. Ser. B 64, 340–353 (2022). https://doi.org/10.1134/S1560090422200040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090422200040