Abstract
The Yubileinoe large gold deposit, located at the southern end of the Magnitogorsk megazone, is the only known representative of the Au–porphyry systems in the Southern Urals. It is genetically related to granitoids formed in a suprasubduction setting under mature oceanic island arc environment/setting. The obtained isotope (Pb–Pb and δ34S) data indicate the input of mineral-forming components into the Au–porphyry system of the deposit, mainly from granitoid melts, confirming a common source of ore material and ore-bearing granitoids. The geochemical and isotopic characteristics of granitoids indicate the leading role in their genesis of the crustal source, which is considered Late Precambrian continental crust.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Deposits of the porphyry family, which play a leading role in the global production of Cu, Mo, Au, Ag, and Re, are formed in various geodynamic settings: suprasubduction, collisional, and postcollisional (Richards, 2009; Sillitoe, 2010; etc.). The diversity of geodynamic settings presupposes the involvement in ore-forming processes of sources of material differing in geochemical nature. Their identification and assessment of their role in the genesis of specific porphyry-type deposits is one of the most pressing issues in the developed genetic models (Sillitoe and Hart, 1984; Bouse et al. 1999; Shafiei, 2010; Plotinskaya et al., 2017a; Shen et al., 2018; etc.).
In existing genetic models describing the formation of porphyry deposits, various sources of metals and volatile components are considered: rocks of subducted oceanic crust, oceanic sediments, metasomatized mantle from the mantle wedge, as well as the asthenospheric and metasomatized subcrustal lithospheric mantle (Sillitoe and Hart, 1984; Bouse et al. 1999; Shafiei, 2010; Richards, 2011; etc.). It is believed that metasomatized mantle of the mantle wedge plays a leading role in the input of metals and volatile components into Cu–porphyry systems associated with a suprasubduction setting (Richards, 2011 and references therein). Conversely, in collisional settings in which Cu–Mo, Mo, and Au porphyry deposits are formed, continental crust makes a significant contribution, but the participation of a mantle source is not excluded.
Several dozens porphyry-type deposits and ore occurrences are known in the Urals (Grabezhev and Belgorodsky, 1992; Seravkin et al., 2011; Seravkin and Kosarev, 2019, Andreev et al., 2018; Minina and Migachev, 2018; Plotinskaya et al., 2017b). Mainly, these are Cu-, Mo- or Au–porphyry and Cu–skarn–porphyry objects. Their formation took place over a long time period (from the Silurian to Late Carboniferous) (Grabezhev et al., 2017; Tessalina and Plotinskaya, 2017) in oceanic island arcs and active continental margin settings, as well as in collision processes (Plotinskaya et al., 2017b). Despite the extensive geological, geochemical, and isotopic dataset on ore mineralization and ore-forming rocks, the question of the source/sources of material in the porphyry systems of the Urals remains controversial (Grabezhev, 2009; Plotinskaya et al., 2017a; etc.).
One of the largest Au–porphyry deposits in the Urals is the Yubileinoe (formerly known as Shekarabulak-II), located in western Kazakhstan and discovered in 1961 (Bespaev et al., 1997). According to the 2015 data from the mining company AO AltynEx for, the balance category C1 + C2 reserves of the deposit amounted to 41 109.5 thousand tons with an average content of 2.07 ppm Au, 2.15 ppm Ag, and 0.156% Cu, i.e., about 85 t Au (AO AltynEx Company, 2015).
To date, the geological structure of the deposit and the mineral and chemical composition of metasomatites and ores have been characterized (Narwaith et al., 1974; Abdulin et al., 1976; Shatov et al., 2014; Plotinskaya et al., 2018; Plotinskaya, 2020; etc.), the geochemical features of ore-bearing granitoids have been studied (Grabezhev and Belgorodsky, 1992), and isotope studies (Nd, Sr, δ18O, δ13C, and δ34S) of ore and gangue minerals, as well as ore-bearing granitoids, have been carried out (Grabezhev et al., 1989; Grabezhev, 2009; Shen et al., 2018).
Based on the results of these studies, it was suggested that the formation of ore-bearing melts occurred at the upper mantle–continental crust boundary (less than 40 km) and is associated with the initial melting of juvenile mantle material, which was accompanied by processes of fractional crystallization and contamination of melts with ancient crustal material (Grabezhev, 2009; Shen et al., 2018). At the same time, researchers have not considered the role of mantle and crustal sources of material that directly took part in ore-forming processes.
In modern studies of porphyry-type deposits, a wide range of isotope geochemistry methods are used to identify sources of ore components, including the Pb–Pb method. Studying the Pb–Pb isotopic characteristics of porphyry mineralization is one of the most effective approaches to reliably identify genetic relationships between igneous rocks and ores and determine the role of mantle and crustal sources in their genesis (Bouse et al. 1999; Kouzmanov et al., 2009; Shafiei, 2010; Borba et al., 2016; Huston et al., 2016; Plotinskaya et al., 2017a; etc.).
In this article, in order to identify the source(s) of lead and sulfur and assess their role in the formation of the Yubileinoe Au–porphyry deposit, we conducted a comprehensive study of the Pb and S isotopic composition in pyrite of the ore mineralization, as well as the Pb–Pb isotopic characteristics of ore-bearing granitoids. Lead isotopic analysis was performed using multiple-collector mass spectrometry with inductively coupled plasma in its high-precision option (MC-ICP-MS). This makes it possible to record small (0.05–0.1%) variations in Pb isotopic ratios in geological objects, reveal correlations against their background, and reliably compare the Pb–Pb isotopic characteristics of ores and igneous rocks.
BRIEF GEOLOGICAL DESCRIPTION OF THE DEPOSIT
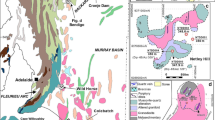
The Yubileinoe deposit is located in the south of the Magnitogorsk volcanic megazone in the Urals in the West Mugodzhar zone (Fig. 1a). Among the country rocks, the most widespread are Early–Middle Devonian volcanic rocks of the tholeiitic series (Mugodzhar Formation), overlain by Middle Devonian volcanosedimentary rocks of the island arc series (Fig. 1b). The volcanic sequences are crosscut by Late Devonian–Early Carboniferous intrusions of mafic, intermediate, and felsic compositions, attributed to the Ayryuk Complex (Narwaith et al., 1974; Abdulin et al., 1976; Grabezhev, 2009; Shatov et al., 2014). The porphyry mineralization is associated with a small (about 400 m in diameter) stock of plagiogranite porphyry with an age of 374 ± 3 Ma (Grabezhev, 2014). In their geochemical characteristics (average contents of Rb = 46 ± 22, Nb = 5.6 ± 1.5, Y = 9.6 ± 2.2, and Ta = 1.1 ± 0.6 μg/g), the rocks are close to granitoids formed both in collisional and volcanic island arc settings (Plotinskaya et al., 2017b; Shen et al., 2018; our unpublished data).
(a) Position of the Yubileinoe porphyry gold deposit (Kazakhstan) on tectonic sketch map of Southern Urals; (b) geological sketch map of the Yubileinoe deposit after (Narwaith et al., 1974). (1) Meso–Cenozoic cover; (2) Pre-Ural foredeep; (3) sialic (a) and volcanic (b) megazones of Southern Urals (WU, Western Ural; M, Magnitogorsk; EU, East Ural volcanic; TU, Trans-Ural); (4) boundaries of Southern Ural megazones; (5) tholeiitic basalts (D1–2); volcano-sedimentary rocks (D2-3) of island arc origin; (6) granitoids ((a) biotite–plagiogranite–porphyry, (b) biotite–amphibole plagiogranite–porphyry) of Ayryuk intrusive complex (D3-C1); (7) ore stockwork.
According to the available geotectonic reconstructions for the South Urals, the formation of the Yubileinoe field could have occurred in the setting of a mature oceanic island arc or in the conditions of an early stage of its collision with the East European continent (Puchkov, 2010; Samygin and Burtman, 2009; etc.).
The ore mineralization is represented by quartz–sulfide and sulfide veinlets 0.5–1 cm thick, which form stockwork zones confined mainly to exo- and endomorphic intrusion contacts. Among the ore minerals, pyrite, chalcopyrite, and magnetite predominate. Bornite, molybdenite, arsenopyrite, sphalerite, galena, and native gold are present in subordinate quantities. Rare minerals include scheelite, sulfosalts, and Bi and Pb sulftellurides. The ore mineralization formed in two stages: early quartz–magnetite–hematite and late chalcopyrite–pyrite (Abdulin et al., 1976; Plotinskaya et al., 2018; Plotinskaya, 2020).
MATERIALS AND METHODS
Sampling
The isotopic composition of lead and sulfur was studied in pyrite from late chalcopyrite–pyrite mineralization with native gold. The pyrite was separated from thin (0.5–1 cm) quartz and quartz–carbonate veinlets localized both in ore-bearing granitoids and in basalts (Figs. 2a, 2d).
Macro- (a–c) and microphotographs (d–f) of ore and rock samples of Yubileinoe porphyry gold deposit (Kazakhstan); (a) quartz veinlets with sulfide mineralization in basalts (sample Yub-1121/300); (b) plagiogranite–porphyry (sample Yub-1105/452); (c) granite micropegmatite in plagiogranite–porphyry (sample Yub-1121/337); (d) pyrite disseminations in vein quartz, reflected light (sample Yub-1121/374); (e) microinclusions of galena and sphalerite in pyrite, BSE image (sample Yub-1121/351); (f) plagioclase phenocrysts among quartz–feldspar groundmass of plagiogranite–porphyry (transmitted light, crossed nicols) (sample Yub-1105/628). Py, pyrite; Gn, galena; Sp, sphalerite; Pl, plagioclase; Mu, muscovite.
The pyrite hosts numerous microinclusions of magnetite, chalcopyrite, and galena (Fig. 2e), as well as Bi and Pb sulfosalts (Plotinskaya, 2020). The analyzed feldspar fractions were represented by plagioclase from plagiogranite–porphyry, which makes up the bulk of the massif, as well as potassium feldspar from late granite micropegmatite veins (Figs. 2b, 2c). The studied plagiogranite–porphyries show signs of metasomatic alteration, which are primarily expressed as sericitization and albitization of plagioclase phenocrysts (Fig. 2f). These processes are manifested most intensely in sample Yub-1105/452.
Analytical Methods
The isotopic composition of Pb and S was studied at the laboratory of isotope geochemistry and geochronology of IGEM RAS.
Pb isotope analysis was performed for monomineralic fractions of pyrite and feldspars of ore-bearing granitoids. The sample weights were 0.02–0.03 g for pyrite and 0.05–0.07 g for feldspar. Before chemical decomposition, pyrite and feldspar were treated with a weak solution of nitric acid (3 and 10%, respectively) to remove lead contamination from the surface of the grain. Chemical preparation of samples, as well as chromatographic separation of Pb, were carried out according to the method described in (Chugaev et al., 2013). The lead fractions obtained after ion exchange chromatography were dissolved in 3% HNO3. The total blank for the chemical procedure did not exceed 0.1 ng Pb. Mass spectrometry measurements were performed on a NEPTUNE multicollector mass spectrometer in the wet plasma mode after (Chernyshev et al., 2007). Solutions with a Pb concentration of 100–400 ng/g were analyzed. Immediately before measurement, the solutions were traced with thallium. Mass-bias correction for the measured Pb isotope ratios was performed using the reference ratio 205Tl/203Tl = 2.3889 ± 1 employing an exponential law. The accuracy of the obtained Pb–Pb data was controlled by the results of parallel analyzes of the Pb isotopic composition standard SRM-981 and the AGV-2 and BCR-1 standard rock samples of the USGS. Total analytical error for 206Pb/204Pb, 207Pb/204Pb and 208Pb/204Pb ratios did not exceed ± 0.02%.
Sulfur isotope analysis was carried out in pyrite samples with a weight of ~0.14 mg. Sulfur was converted to gaseous SO2 using an FlashHT 1112 elemental analyzer at 1020°C in a reactor filled with Cu0 and WO3. Samples and standards in tin capsules were sequentially introduced into the reactor using an autosampler. The sulfur isotopic composition in SO2 gas was measured by CF-IRMS on a DELTAV + mass spectrometer (Finnigan). δ34S was calibrated against three international standards, IAEA-S-1 (–0.3), IAEA-S-3 (–32.55‰) and NBS-127 (+21.1 ‰), which were analyzed simultaneously with the samples. The results obtained are expressed in the international scale V-CDT (Vienna Canyon Diablo Troilite):
The reproducibility of the results in a series of parallel measurements of standard samples was ±0.25 ‰ (± 1σ).
In order to calculate the initial Pb isotopic ratios in the same weighed portions of monomineralic fractions for which the Pb isotopic composition was measured, data on the concentrations of Pb, Th, and U were obtained. The concentrations of these elements were measured by ICP-MS from solutions on an iCAP SQ ICP-MS inductively coupled plasma mass spectrometer (Thermo Scientific) at the laboratory of isotopic and elemental analysis of IGiNT KFU. The error in determining the Pb, Th, and U contents in the samples, estimated from the results of systematic analyses of standard rock samples BHVO-2 and AGV-2 of the USGS, did not exceed ±3% (±2SD).
RESULTS
Table 1 lists the measured Pb isotopic ratios, δ34S values, and data on the Pb, Th, and U contents in pyrite and feldspars.
U–Th–Pb Systematics of Granitoid Pyrite and Feldspars
The Pb isotopic composition, as well as data on the Pb, Th, and U concentrations, were obtained for six pyrite samples from the ore stockwork and four samples of feldspars of ore-bearing granitoids from the Yubileinoe deposit. Pyrites from sulfide veinlets are generally characterized by high Pb contents, which vary in a wide range from 9.8 to 1840 ppm. Such a significant heterogeneity of the studied pyrite samples in Pb content is due to the uneven presence of lead and bismuth sulftellurides microinclusions in the mineral (Plotinskaya, 2020), as well as galena (Fig. 2e). Conversely, the Th and U concentrations in pyrite turned out to be low. For most of the samples, the Th content is below the detection limit, while the maximum value was 0.03 ppm. The U content is slightly higher than the detection limit and lies in the range of values from 0.01 to 0.07. Varying high Pb concentrations at low and sufficiently sustained Th and U contents determine low values of the ratios 232Th/204Pb and 238U/204Pb, which do not exceed 0.007 and 0.052, respectively. Taking into account these data, the correction of the measured values of isotope ratios 206Pb/204Pb and 208Pb/204Pb for an age of 374 Ma does not exceed 0.02%, i.e., within the analytical error of measuring these ratios.
Feldspars of ore-bearing granitoids have Pb contents typical of these minerals varying from 9.1 to 53 μg/g. The highest value was obtained for the fraction of potassium feldspar sampled from late granite micropegmatite. The measured Th and U concentrations vary from 0.3 to 2.5 and from 0.6 to 2.1 ppm, respectively. The elevated Th and U contents, in turn, determine the high 232Th/204Pb and 238U/204Pb ratios in feldspars. In this case, the 232Th/204Pb values vary within narrow limits (2.1–3.1), while for 238U/204Pb, the range of Pb ratios is several times wider, from 1.3 to 15. As a consequence, the correction of the 208Pb/204Pb and 206Pb/204Pb ratios for an age of 374 Ma for all feldspar samples significantly exceeds the analytical error, 0.15 and 5%, respectively. After correction, the isotopic composition of Pb in feldspars turned out to be more homogeneous, and the values of isotopic ratios change in the following ranges: for 206Pb/204Pb—18.43–18.49, 207Pb/204Pb—15.652–15.659, 208Pb/204Pb—38.351–38.357. In this case, geochemically significant variations (i.e., exceeding the analytical error) are only variations in the ratio 206Pb/204Pb. In the case of a the 207Pb/204Pb and 208Pb/204Pb ratios, the scatter of values, estimated from the value of the variation coefficient, is equal to or less than the analytical error. Despite such a small scale of variations, it can be seen from the obtained Pb–Pb data that the most radiogenic initial isotopic composition of lead in terms of isotope content 206Pb is found in late pegmatoid veins, while in ore-bearing plagiogranite–porphyry, the 206Pb/204Pb ratio is systematically lower and lies in a narrow range from 18.425 to 18.443, for which the difference in extreme values is only 0.05%.
δ34S Values in Pyrite
The sulfur isotopic composition in the studied pyrite samples is relatively uniform. High positive δ34S values were obtained for this mineral, varying from +7.6 to +9.7‰. In this case, no correlation was found between the δ34S value in pyrite and geological and/or mineralogical characteristics of the sample. The data presented in this work are consistent with previously published results of similar studies (Grabezhev et al., 1989), who reported that δ34S values for pyrite from the ore stockwork of the Yubileinoe deposit vary from +8.5 to +9.0‰ (three samples).
DISCUSSION
Sources of Pb in Ores and Rocks
Spatial association of ore and granitoids at the Yubileinoe deposit suggests their genetic relationship and a single source of material (Grabezhev, 2009; Shatov et al., 2014; etc.). Therefore, it is of interest to compare the isotopic composition of Pb ores with that in granitoids and to assess the role of felsic magmatic melts in Pb input to the mineral-forming system of the deposit. Figure 3 shows that pyrite from the ore stockwork and plagioclase of plagiogranite–porphyry are very similar in Pb isotopic composition. Some of the points of pyrite fall in the field of ore-bearing granitoids. However, despite the similarity of the Pb isotopic ratios of ores and igneous rocks and the small scale of variations, there are distinct differences: the points of ore lead are systematically shifted to the left of the of isotopic composition field of Pb granitoids and form short trends in both diagrams, which, based on their position, can be interpreted as mixing trends. The relationship between the trends and isotopic composition fields of Pb granitoids indicates that felsic magmatic melts were one of the sources of lead in ores and their contribution was decisive. At the same time, Pb input from another source has also been established. Its contribution was insignificant, and the lead in it had a reduced 206Pb content and, conversely, elevated concentrations of 207Pb and 208Pb.
Pb–Pb diagrams for Pb isotopic composition of pyrite and feldspars of granitoids from Yubileinoe gold–porphyry deposit (Kazakhstan). Diagrams show Pb isotopic ratios corrected for 374 Ma. Gray line shows trend of isotopic composition of ore Pb; dashed line shows isotopic composition field of Pb plagioclases of plagiogranite–porphyry ore-bearing stock. Analytical errors: for pyrite, ±0.01% (SD); for feldspars, ±0.015% (SD).
According to the Stacy–Kramers model (Stacey and Kramers, 1975), the source of material of ores and magmatic melts at the Yubileinoe deposit was characterized by elevated values of with respect to the mean crustal values µ2 (238U/204Pb) = 9.92 ± 0.01 and ω2 (232Th/204Pb) = 38.03 ± 0.10. The values of the Pb–Pb model age (Тm = 260–280 Ma) reflecting the estimated time of Pb separation from the U–Th–Pb isotope system of the source proved, on average, 100 Ma younger than the geological age of the deposit. This discrepancy indicates that shortly before Pb separation from the U–Th–Pb system as a result of magmatic and ore-forming processes, there was a change (increase) in the U/Pb ratio in the source, e.g., due to metasomatic or metamorphic alteration of crustal rocks.
On the plots, point corresponding to K-feldspar of late pegmatoid veins is to the right of the field of ore-bearing plagiogranite–porphyry. The observed ratio of the Pb isotopic composition of rocks allows us to conclude that the studied granitoids had a common source of magmatic melts in their U–Th–Pb characteristics. In this case, the shift with respect to 206Pb/204Pb in granite micropegmatites is explained by accumulation of 206Pb in the source during the time that separates the formation of the plagiogranite–porphyry stock and intersecting granite micropegmatite veins. Based on this assumption, it is possible to estimate the duration of the evolution of the Pb isotopic composition in the source, taking for μ2 a value of 9.92 and ∆ (206Pb/204Pb) = 0.061. From the calculations, it follows that the formation of late granite micropegmatite veins was separated from plagiogranite–porphyry by no more than 40 Ma.
Figure 4 shows the average crustal growth curve according to the Stacy–Kramers model and the curves of evolution of the isotopic composition of Pb in various crustal reservoirs of the Earth according to the Zartman–Doe model (Zartman and Doe, 1981). For comparison, the isotopic composition fields of Pb ore of the Silurian epithermal and porphyry deposits of the East Ural volcanic megazone and Early Devonian Cu–porphyry deposits of the Magnitogorsk megazone are also plotted (Plotinskaya et al., 2017a; Chugaev et al., 2019). Their formation is associated with oceanic island arcs of different ages. Additionally, Pb–Pb data are given for suprasubduction Late Paleozoic (D3–C1) granitoids of the Chelyabinsk pluton (East Ural megazone) (Plotinskaya et al., 2017a) and the Mindyak gold deposit (Magnitogorsk megazone), close to them in age, localized among rocks of a black shale formation (Chugaev and Znamensky, 2018). The latter show the Pb–Pb isotopic characteristics of the regional crustal reservoir for the Southern Urals in the Late Devonian–Early Carboniferous, to which the Yubileinoe deposit is similar in age.
Pb–Pb diagrams for porphyry and epithermal deposits and rocks of Southern Urals. Diagrams show Pb isotope ratios corrected for age of deposits. S-K, evolutionary curves according to Stacy–Kramers model (Stacey and Kramers, 1975); solid lines, curves of Pb isotopic composition evolution in crustal geochemical reservoirs of Earth according to Zartman–Doe model (Zartman and Doe, 1981).
On both graphs, the points of the isotopic composition of Pb ores and granitoids of the Yubileinoe deposit form compact fields (Figs. 4a, 4b). On the diagram with “uranogenic” Pb isotopes (Fig. 4b), the points are located near the upper crustal evolutionary curve, while in 206Pb/204Pb–208Pb/204Pb coordinates (Fig. 4a), they lie above the average crustal growth curve and crustal reservoir curves (upper crust and orogen). The points corresponding to the Yubileinoe deposit are located outside the fields of the Pb isotopic composition of Cu–porphyry and epithermal deposits of the East Ural volcanic and Magnitogorsk megazones, for which the participation of a mantle source has been suggested (Grabezhev, 2009; Plotinskaya et al., 2017a). The Yubileinoe deposit also has Pb–Pb isotopic characteristics similar to the suprasubduction granitoids of the Chelyabinsk pluton and gold mineralization of the Mindyak deposit, in which lead had predominantly crustal origin.
These features, as well as the values of the model parameters (μ2, ω2), indicate that the lead of ore and rocks of the Yubileinoe deposit had a crustal source. In turn, the ratio of the Pb isotopic composition fields of the Yubileinoe deposit and porphyry-epithermal deposits of the East Ural and Magnitogorsk megazones indicate the predominant role of crustal lead in the Au–porphyry mineralization. This conclusion agrees with the results of an Sm–Nd study of ore-bearing plagiogranite-porphyry and their chemical characteristics (low Cr (7–12 ppm) and Ni (8–15 ppm) concentrations and low Nb/Ta = 8.1–8.7) (Grabezhev, 2009; Shen et al., 2018). The obtained negative of εNdt (–2.6…–2.9) values and Proterozoic (1.3–1.4 Ga) model (TDM2) Nd ages (Shen et al., 2018) indicate the formation of granitoid melts as a result of melting of Proterozoic crust.
Sources of Sulfur
For the Yubileinoe deposit, a narrow range of variations in δ34S (+7.6 to +9.7‰) has been established that is typical of porphyry deposits in various regions of the world, (He et al., 2020; Hutchison et al., 2020). In the Southern Urals, within individual porphyry deposits, variations in δ34S also do not exceed 2–3‰; however, these values themselves (–1.5 to +5.7‰) are significantly lower than those obtained for the Yubileinoe deposit (Grabezhev et al., 1989). Thus, the Yubileinoe deposit is characterized not only by homogeneous sulfur isotopic characteristics, determined by its fluid transfer (Dubinina et al., 2010), but also elevated δ34S values, which is unusual compared to analogous fields. If fluid transfer of ore components for porphyry-type deposits is not in doubt, then the mechanism leading to deposition of isotope-enriched 34S sulfides requires discussion, since at least two reasons of the appearance of similar sulfur isotopic characteristics are possible. Fundamentally, these reasons differ in the role of host rocks as one of the sources of sulfur in the ore-forming fluid. Isotope-weighted reduced sulfur in the ore-forming system can be either a product of sulfur entrainment from surrounding rocks, or the result of a change in redox conditions in the evolution of the fluid system.
In the first case, with an insignificant role of sulfur redox processes during its extraction and further evolution of the fluid system, it is possible to estimate the approximate amount of sulfur extracted from the surrounding rocks. For δ34S of the magmatic source of sulfur close to zero and a δ34S value for sulfur of the host Devonian volcano-sedimentary rocks corresponding to the sulfur isotopic composition of Devonian marine sulfate (23‰, (Canfield and Farquhar, 2009)), it is necessary to extract about 40% of sulfur from the surrounding rocks in the fluid in order to yield the observed range of δ34S in sulfides of the Yubileinoe deposit.
An alternative variant implies a change in the sulfur oxidation state at different stages in the evolution of the fluid ore-forming system. In the absence of sulfur from surrounding rocks, the possible formation of sulfide minerals with high δ34S values will depend on the form of sulfur in the magmatic melt, which is determined primarily by the oxygen fugacity. If fO2 exceeds the values that determine the sulfide–sulfate barrier (Markl et al., 2010), then oxidized sulfur predominates in the melt. In this case, the separation of the fluid containing SO2 with a sulfur isotopic composition δ34S (SO2)0 and its further cooling with an increase in the Н2S/SO2 ratio (Rye, 2005; Richards, 2011) cannot lead to the appearance of δ34S sulfide sulfur values exceeding the initial values of δ34S (SO2)0. Since 34S in the entire temperature range is distributed in favor of oxidized sulfur (Eldridge et al., 2016), sulfides precipitated from such a fluid usually have zero or small negative δ34 S (Rye, 2005; Richards, 2011; etc.).
However, if fO2 ensures the presence of sulfur in the melt in the form of an S2– particle, and sulfur leaves the fluid in the form of SO2, the situation may be the opposite. At the moment of fluid separation, sulfur fractionation occurs, which is determined by the temperature of the process, as a result of which the δ34S (SO2)0 values can vary within fairly wide limits. Figure 5 shows the calculation of δ34S (SO2)0 values depending on the temperature of fluid separation and initial isotopic composition of magmatic sulfur (for the calculation, the initial values were δ34S = 0, +2, and +4‰). The vertical dotted line limits the range of δ34S values observed in sulfides of the Yubileinoe deposit. As follows from the calculation from the thermometric dependences (Eldridge et al., 2016), for zero values of δ34S of magmatic sulfur, fluid separation could have occurred in the range 300–370°C. For magmatic sulfur with δ34S = +2‰, the fluid could have separated at 370–480°С, and for δ34S = +4‰, already at 480–670°C. In this model, it is assumed that as the fluid cools, a rapid change in P–T conditions occurs, as a result of which nearly all oxidized sulfur is quantitatively reduced and does not undergo subsequent exchange with other sulfur-containing phases. This mechanism was proposed in a study of sulfur isotopic variations in ore sulfides of the Akchatau deposit (Dubinina et al., 1995). Its realization undoubtedly requires fulfillment of a number of conditions, first of all, predominance of S2–.
Calculated lines of variation of δ34S (SO2)0 as function of temperature of separation of magmatic fluid. Numerals on lines are initial δ34S values of magmatic sulfur. Dotted line, range of δ34S in sulfides of Yubileinoe deposit. Calculation was done according to equations after (Eldridge et al., 2016).
The low sulfur content in apatite from granites of the Yubileinoe stock and, conversely, high sulfur content in biotite (Grabezhev and Voronina, 2012) suggests that the predominant form of sulfur in the melt was S2– (Tang et al., 2020). In turn, infiltration of the fluid into heated mafic host rocks containing a significant amount of ferrous iron should lead to abiogenic sulfate reduction, i.e., quantitative conversion of all oxidized sulfur contained in the fluid into reduced form (Canfield and Farquhar, 2009). Thus, the above-described mechanism for the occurrence of elevated δ34S values in sulfides could have occurred during formation of the Yubileinoe deposit.
It should be noted that we should not exclude a combination of the two above-mentioned mechanisms of the formation of sulfides with high δ34S at the deposit. During formation of an ore stockwork in tholeiitic basalts during thermogenic reduction of sulfur, its partial extraction from the host rocks could occur. A similar process usually takes place during thermogenic reduction of seawater sulfate in convective hydrothermal systems (Dubinina et al., 2020).
The participation of material from a nonmagmatic source in ore-forming processes is indicated by the relationship between the δ34S values with the 207Pb/204Pb and 208Pb/204Pb ratios (Figs. 6a, 6b). From the direction of the coordinated change in these values, it follows that the isotopic composition of sulfur in this source should be characterized by the value δ34S < 7.6‰. The position of the Pb–Pb trends on the isotope diagrams (Figs. 4a, 4b) suggests that the additional Pb input came from a source with high values of µ2 (>9.94) and ω2 (>38.2).
Comparison of δ34S values and 208Pb/204Pb (a) and 207Pb/204Pb (b) ratios of pyrite from Yubileinoe gold–porphyry deposit (Kazakhstan). Diagrams show Pb isotopic ratios corrected for 374 Ma. Trends of δ34S and 207Pb/204Pb and 208Pb/204Pb ratios are shown in gray. Analytical uncertainties (±0.01%, SD) are indicated for Pb isotopic ratios. For δ34S, error is less than size of symbol.
The role of a Crustal Source in the Gold Ore Mineralization
Gold-bearing porphyry mineralization in most deposits is associated with acidic calc-alkaline and subalkaline melts, which formed in collisional or suprasubduction (Andean type) settings as a result of melting of continental crust. Compared to typical suprasubduction Cu–porphyry deposits of oceanic island arcs, the fluid in such porphyry systems is in a more oxidized state, which facilitates Au transport from the source enriched with ore components and its deposition at the mid- and upper levels of the lithosphere (Richards, 2011). In this case, as a result of interaction of ore-bearing melts and fluids with host rocks, continental material crust is entrained, proved by the results of a lead isotope study of Cu– (Au)–Mo–porphyry deposits in different regions of the world, differing in age and geotectonic formation setting (Kouzmanov et al., 2009; Chugaev et al., 2013; Borba et al., 2016; Zang et al., 2016; etc.). As a rule, continental crust of the tectonic block hosting the deposit is considered the crustal source of lead in such studies. The contribution of material from an enriched mantle source has also been established, although its contribution to the overall lead input balance is of lesser importance. A distinctive feature of the Yubileinoe deposit is the dominant role of a crustal source for ore and magmatic lead. This conclusion, as well as the available geochemical and isotope characteristics of ore-bearing granites, which are close to syncollisional granitoids, suggest that the formation of the Yubileinoe deposit is associated with the onset of collisional processes in the Southern Urals in the Late Devonian.
CONCLUSIONS
Generalization of the available isotope dataset for ores and rocks of the Yubileinoe Au–porphyry deposit, including the present results of, allows us to conclude the following.
(1) The input of Pb to the Au–porphyry system of the Yubileinoe deposit mainly originated from silicic magmas that formed plagiogranite–porphyries. Pb–Pb isotope data directly confirm a common source for ores and ore-bearing granitoids, which agrees with the previously proposed model of genesis of the Yubileinoe deposit (Grabezhev, 2009). As well, the entrainment of lead in ore-forming processes from another source has been established for the first time, which could have been the host rocks. However, the contribution of this source to the overall balance of ore lead was minimal.
(2) The formation of ore-generating granitoid melts occurred due to melting of relatively ancient (Late Precambrian) continental crust, which included metamorphic rocks.
The latter conclusion does not agree with the concept of generation of ore-bearing melts at the upper mantle–continental crust boundary due to melting of the mafic substrate (Grabezhev, 2009; Shen et al., 2018). Our results, conversely, indicate the dominant role of a crustal source in the genesis of Au–porphyry mineralization, which is considered to be Late Precambrian crust. Its melting could have been caused by collisional processes, which began in the region in the Late Devonian–Early Carboniferous (Puchkov, 2010, Samygin and Burtman, 2009). In turn, there is no isotopic evidence of a significant contribution of juvenile mantle material.
Change history
28 September 2021
An Erratum to this paper has been published: https://doi.org/10.1134/S1075701521300012
REFERENCES
Abdulin, A.A., Baidil’din, E.A., Kasymov, M.A. Matvienko, V.N., Tapalov, E.D., and Tel’guziev, A.T., Metallogeniya Mugodzhar (Metallogeny of Mugodzhary), Alma-Ata: Nauka KazSSR, 1976.
Andreev, A.V., Girfanov, M.M., Kulikov, D.A., Migachev, I.F., Minina, O.V., Avilova, O.V., Krasnosel’skikh, A.A., Starostin, I.A., and Cheremisin, A.A., Ore districts with copper-porphyry mineralization: prospective mineral-raw cooper base of the south Urals, Otechestvennaya Geol., 2018, no. 4, pp. 3–17.
Bespaev, Kh.A., Globa, V.A., Abishev, V.M., Gulyaeva, N.Ya., Mestorozhdeniya zolota Kazakhstana. Spravochnik (Gold Deposits of Kazakhstan. A Manual), Alma-ata: Informatsionno-analiticheskii tsentr geologii, ekologii i prirodnykh resursov Respubliki Kazakhstan, 1997.
Borba, M.L., Junior, F.C., Kawashita, K., Takehara, L., Babinski, M., and Bruckman, M., The Bajo de la Alumbrera and Agua Rica Cu–Au (mo) porphyry deposits of Argentina: genetic constraints on ore formation and sources based on isotope signatures, Ore Geol. Rev, 2016, vol. 75, pp. 116–124.
Bouse, R.M., Ruiz, J., Titley, S.R., Tosdal, R.M., and Wooden, J.L., Lead isotope compositions of Late Cretaceous and early Tertiary igneous rocks and sulfide minerals in Arizona; implications for the sources of plutons and metals in porphyry copper deposits, Econ. Geol., 1999, vol. 94, no. 2, pp. 211–244.
Boyce, A.J., The sulfur isotope evolution of magmatic–hydrothermal fluids: insights into ore-forming processes, Geochim. Cosmochim. Acta, 2020, vol. 288, pp. 176–198.
Canfield, D.E. and Farquhar, J., Animal evolution, bioturbation and the sulfate concentration of the oceans, Proc. Natl. Acad. Sci. USA, 2009, vol. 106, pp. 8123–8127.
Chernyshev, I.V., Chugaev, A.V., and Shatagin, K.N., High-precision Pb isotope analysis by multicollector-ICP-mass-spectrometry using 205Tl/203Tl normalization: optimization and calibration of the method for the studies of Pb isotope variations, Geochem. Int., 2007, vol. 45, no. 11, pp. 1065–1076.
Chugaev, A.V. and Znamenskii, S.E., Lead isotope characteristics of the Mindyak gold deposit, Southern Urals: evidence for the source of metals, Geol. Ore Deposits, 2018, vol. 60, no. 1, pp. 52–61.
Chugaev, A.V., Chernyshev, I.V., Bortnikov, N.S., Kovalenker, V.A., Kiseleva, G.D., and Prokof’ev, V.Yu., Lead isotope ore provinces of Eastern Transbaikalia and their relationships to regional structures: results of high-precision MC-ICP-MS study of Pb isotopes, Geol. Ore Deposits, 2013, vol. 55, no. 4, pp. 245–255.
Chugaev, A.V., Chernyshev, I.V., Lebedev, V.A., and Eremina, A.V., Lead isotope composition and origin of the Quaternary lavas of Elbrus Volcano, the Greater Caucasus: high-precision MC-ICP-MS data, Petrology, 2013, vol. 21, no. 1, pp. 16–27.
Chugaev, A.V., Plotinskaya, O.Yu., Gareev, B.I., Batalin, G.A., Mandzhieva, G.V., and Sadasyuk, A.S., Sources of ore matter of Cu-porphyry and Cu-skarn deposits of the South Urals: results of first system Pb-Pb isotope studies, Materialy XXII simpoziuma po geokhimii izotopov imeni akademika A.P. Vinogradova (Proc. 27th A.P. Vinogradov Symposium on Isotope Geochemistry), 2019, pp. 489-496.
Dubinina, E.O., Suvorova, V.A., and Shapovalov, Yu.B., Sulfur isotopes in the ore genesis of the Akchatau deposit (Kazakhstan), Geokhimiya, 1995, vol. 341, no. 1, pp. 102–105.
Dubinina, E.O., Ikonnikova, T.A., and Chugaev, A.V., Heterogeneity of the sulfur isotopic composition of pyrite at the Sukhoi Log deposit and its controlling factors, Dokl. Earth Sci., 2010, vol. 435, no. 6, pp. 1665–1669.
Dubinina, E.O., Bortnikov, N.S., Stavrova, O.O., and Kossova, S.A., Sulfur isotope fractionation during sulfide generation in the hydrothermal submarine systems: the case of Logatchev, Krasnov, and Rainbow hydrothermal fields, Mid-Atlantic Ridge, Geol. Ore Deposits, 2020, vol. 62, no. 5, pp. 391-413.
Eldridge, D.L., Guo, W., and Farquhar, J., Theoretical estimates of equilibrium sulfur isotope effects in aqueous sulfur systems: highlighting the role of isomers in the sulfite and sulfoxylate systems, Geochim. Cosmochim. Acta, 2016, vol. 195, pp. 171–200.
Godovoi otchet za 2015 g. (Annual Report for 2015), JSC AltynEx Company, 2015. https://kase.kz/files/emitters/ ATEC/atecp_2015_ rus.pdf. Cited May 01, 2020.
Grabezhev, A.I., Sr-Nd-COHS isotope-geochemical characteristics of copper-porphyry fluid-magmatic systems of the South Urals: probable ore sources, Litosfera, 2009, no. 6, pp. 66–89.
Grabezhev, A.I., The Yubileinoe porphyry Cu–Au deposit (South Urals, Russia): SHRIMP-II U–Pb zircon age and geochemical features of ore-bearing granitoids, Dokl. Earth Sci., 2014, vol. 454, no. 1. C. 72–75.
Grabezhev, A.I. and Belgorodskii, E.A., Produktivnye granitoidy i metasomatity medno-porfirovykh mestorozhdenii (Productive Granitoids and Metasomatites of Copper-Porphyry Deposits), Yekaterinburg: IGG UrO RAN, 1992.
Grabezhev, A.I., and Voronina, L.K., Sulfur in apatite from rocks of the copper-porphyry systems of the Urals, Ezhegodnik-2011, 2012, vol. 159, pp. 68–70.
Grabezhev, A.I., Sotnikov, V.I., and Chashchukhina, V.A., Sulfur isotope composition of sulfides from the copper–porphyry deposits of the Urals, Geokhimiya, 1989, no. 10, pp. 1508–1511.
Grabezhev, A.I., Shardakova, G.Yu., Ronkin, Yu.L., and Azovskova, O.B., Systematics of U-Pb ages of zircons from granitoids of the copper–porphyry deposits of the Urals, Litosfera, 2017, vol. 17, no. 5, pp. 113–126. https://doi.org/10.24930/1681-9004-2017-17-5-113-126
He, Z., Zhang, X., Deng, X., Hud, H., Li, Y.YuH., Archer, C., Li, J., and Huang, F., The behavior of Fe and S isotopes in porphyry copper systems: constraints from the Tongshankou Cu–Mo deposit, Eastern China, Geochim. Cosmochim. Acta, 2020, vol. 270 P, pp. 61–83.
Hutchison, W., Finch, A.A., and Boyce, A.J., The sulfur isotope evolution of magmatic-hydrothermal fluids: insights into ore-forming processes, Geochem. Cosmochim. Acta, 2020, vol. 288, pp. 176–198.
Huston, D.L., Champion, D.C., Mernagh, T.P., Downes, P.M., Jones, P., Carr, G., Forster, D., and David, V., Metallogenesis and geodynamics of the Lachlan Orogen: new (and old) insights from spatial and temporal variations in lead isotopes, Ore Geol. Rev., 2016, vol. 76, pp. 257–267.
Kouzmanov, K., Moritz, R., von Quadt, A., Chiaradia, M., Peytcheva, I., Fontignie, D., Ramboz, C., and Bogdanov, K., Late Cretaceous porphyry Cu and epithermal Cu–Au association in the southern Panagyurishte District, Bulgaria: the paired Vlaykov Vruh and Elshitsa deposits, Miner. Deposita, 2009, vol. 44, no. 6, pp. 611–646.
Markl, G., Marks, M.A.W., and Frost, B.R., On the controls of oxygen fugacity in the generation and crystallization of peralkaline melts, J. Petrol., 2010, vol. 51, no. 9, pp. 1831–1847.
Minina, O.V. and Migachev, I.F., Copper porphyry provinces and zones of the South Urals: prediction–metallogenic zoning, Otechestvennaya Geol., 2018, no. 4, pp. 3–17.
Narvait, G.E., Rudenko, B.M., Miroshnichenko, L.A., and Zhukov, N.M., Mednoe orudenenie Mugodzhar (Copper Mineralization of Mugodzhary), Alma-Ata: Nauka KazSSR, 1974.
Plotinskaya, O.Yu., Mineralogy of noble-metal metals in the ores of the Yubileinoe gold-porphyry deposit, Kazakhstan, Mineralogiya, 2020, vol. 6, no. 3, pp. 44-53.
Plotinskaya, O.Y., Chugaev, A.V., and Seltmann, R., Lead isotope systematics of porphyry-epithermal spectrum of the Birgilda–Tomino ore cluster in the South Urals, Russia, Ore Geol. Rev., 2017a, vol. 85, pp. 204–215. https://doi.org/10.1016/j.oregeorev.2016.09.006
Plotinskaya, O.Y., Grabezhev, A.I., Tessalina, S., Seltmann, R., Groznova, E.O., and Abramov, S.S., Porphyry deposits of the Urals: geological framework and metallogeny, Ore Geol. Rev., 2017b, vol. 85, pp. 153–173. https://doi.org/10.1016/j.oregeorev.2016.07.002
Plotinskaya, O.Yu., Baksheev, I.A., and Minervina, E.A., REE distribution in scheelite from the Yubileinoe porphyry gold deposit, South Urals: evidence from LA-ICP-MS data, Geol. Ore Deposits, 2018, vol. 60, no. 4, pp. 355–364.
Puchkov, V. N., Geology of the Urals and Cis-Urals: actual questions of stratigraphy, tectonics, and metallogeny, Ural’sk Geol. Zh., 2010, no. 3, pp. 80–84.
Richards, J.P., Postsubduction porphyry Cu–Au and epithermal Au deposits: products of remelting of subduction-modified lithosphere, Geology, 2009, vol. 37, p. 247–250.
Richards, J.P., Magmatic to hydrothermal metal fluxes in convergent and collided margins, Ore Geol. Rev., 2011, vol. 40, pp. 1−16.
Rye, R.O., A review of the stable-isotope geochemistry of sulfate minerals in selected igneous environments and related hydrothermal systems, Chem. Geol., 2005, vol. 215, pp. 5–36.
Samygin, S.G. and Burtman, V.S., Tectonics of the Ural Paleozoides in comparison with the Tien Shan, Geotectonics, 2009, vol. 43, no. 2, pp. 133–152.
Seravkin, I.B. and Kosarev, A.M., Southern Urals and Rudny Altai: a comparative paleovolcanic and metallogenic analysis, Geol. Ore Deposits, 2019, vol. 61, no. 2, pp. 99–117.
Seravkin, I.B., Minibaeva, K.R., and Rodicheva, Z.I., Copper–porphyry mineralization of the South Urals: a review, Geol. Sb., 2011, no. 9, pp. 186–200.
Shafiei, B., Lead isotope signatures of the igneous rocks and porphyry copper deposits from the Kerman Cenozoic magmatic arc (SE Iran), and their magmatic-metallogenetic implications, Ore Geol. Rev., 2010, vol. 38, nos. 1–2, pp. 27−36.
Shatov, V.V., Moon, C.J., and Seltmann, R., Discrimination between volcanic associated massive sulphide and porphyry mineralisation using a combination of quantitative petrographic and rock geochemical data: a case study from the Yubileinoe Cu–Au deposit, western Kazakhstan, J. Geochem. Explor., 2014, vol. 147, pp. 26–36.
Shen, P., Pan, H., Hattori, K., Cooke, D.R., and Seitmuratova, E., Large Paleozoic and Mesozoic porphyry deposits in the Central Asian Orogenic Belt: geodynamic settings, magmatic sources, and genetic models, Gondwana Res., 2018, vol. 58, pp. 161–194.
Sillitoe, R.H., Porphyry copper systems, Econ. Geol., 2010, vol. 105, pp. 3–41.
Sillitoe, R.H. and Hart, S.R., Lead-isotopic signatures of porphyry copper deposits in oceanic and continental settings, Colombian Andes, Geochim. Cosmochim Acta, 1984, vol. 48, no. 10, pp. 2135–2142.
Stacey, J.S. and Kramers, I.D., Approximation of terrestrial lead isotope evolution by a two-stage model, Earth Planet. Sci. Lett., 1975, vol. 26, no. 2, pp. 207–221.
Tang, M., Lee, Cin-TyA., Ji, W.-Q., Wang, R., and Costin, G., Crustal thickening and endogenic oxidation of magmatic sulfur, Sci. Adv., 2020, vol. 6, no. 31.
Tessalina, S. and Plotinskaya, O.Y., Silurian to Carboniferous Re–Os molybdenite ages of the Kalinovskoe, Mikheevskoe and Talitsa Cu- and Mo porphyry deposits in the Urals: implications for geodynamic setting, Ore Geol. Rev., 2017, vol. 85, pp. 174–180.https://doi.org/10.1016/j.oregeorev.2016.09.005
Zartman, R.E. and Doe, B.R., Plumbotectonics - the model, Tectonophysics, 1981, vol. 75, pp. 135–162.
Zhang, F.F., Wang, Y.H., and Liu, J.J., Fluid inclusions and H–O–S–Pb isotope systematics of the Baishan porphyry Mo deposit in Eastern Tianshan, China, Ore Geol. Rev., 2016, vol. 78, pp. 409–423.
Funding
The research was supported by the Russian Foundation for Basic Research (project no. 19-05-00344—study of the S and Pb isotopic composition) and the Ministry of Science and Higher Education of the Russian Federation (contract no. 14.Y26.31.0029) as part of implementation of Resolution no. 220 of the RF Government (geochemical study of minerals).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chugaev, A.V., Plotinskaya, O.Y., Dubinina, E.O. et al. Crustal Source of Pb and S at the Yubileynoe Porphyry Gold Deposit (Southern Urals, Kazakhstan): High Precision Pb–Pb and δ34S Data. Geol. Ore Deposits 63, 173–184 (2021). https://doi.org/10.1134/S107570152103003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107570152103003X