Abstract
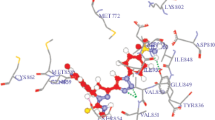
A series of benzimidazole-tethered pyrrolones and N-benzylpyrrolones were synthesized, characterized, and explored for their in vitro anti-proliferative activities. The cytotoxicity of the synthesized compounds was evaluated against three cancer cell lines, A549, MCF7, and DU145. (E)-5-(1H-Benzimidazol-2-yl)-3-(3,4,5-trimethoxbenzylidene)-1H-pyrrol-2(3H)-one having three methoxy groups at 3,4,5-positions exhibited excellent activity against A549, MCF7, and DU145 cancer cell lines with IC50 values of 8.3±0.53, 7.2±1.42, and 7.7±0.13 µM, respectively. Another pyrrolone derivative with one hydroxy and one methoxy substituents, (E)-5-(1H-benzimidazol-2-yl)-3-(4-hydroxy-3-methoxybenzylidene)-1H-pyrrol-2(3H)-one, also displayed very good activity against A549, MCF7, and DU145 cell lines with IC50 values of 9.6±0.12, 7.3±0.24, and 8.7± 0.24 µM, respectively. Molecular docking study revealed that all compounds fit into the pocket of VEGFR-2 within the key amino acid residues Glu885, Cys919, and Asp1046. The docking scores and binding energies were very consistent with the experimental anticancer activity. Pharmacokinetic (ADME) parameters of the potent derivatives were also found to be within an acceptable range. It could be concluded that benzimidazole-linked pyrrolones are more potent than their benzylpyrrolone analogs, and therefore this class of compounds could be explored further for the development of potent anticancer agents.








Similar content being viewed by others
REFERENCES
Ahn, Y. and Jun, Y., Early Hum. Dev., 2007, vol. 83, p. 255. https://doi.org/10.1016/j.earlhumdev.2006.05.022
Rashid, M., Husain, A., and Mishra, R., Eur. J. Med. Chem., 2012, vol. 54, p. 855. https://doi.org/10.1016/j.ejmech.2012.04.027
Hadoux, J. and Schlumberger, M., Best Pract. Res., Clin. Endocrinol. Metab., 2017, vol. 31, p. 335. https://doi.org/10.1016/j.beem.2017.04.009
Karabajakian, A., Toussaint, P., Neidhardt, E.M., Paulus, V., Saintigny, P., and Fayette, J., Anticancer Drugs, 2017, vol. 28, p. 362. https://doi.org/10.1097/CAD.0000000000000480
Chaffer, C.L. and Weinberg, R.A., Science, 2011, vol. 331, p. 1559. https://doi.org/10.1126/science.1203543
Steeg, P.S. and Theodorescu, D., Nat. Clin. Pract. Oncol., 2008, vol. 5, p. 206. https://doi.org/10.1038/ncponc1066
Jukić, M., Rastija, V., Opačak-Bernardi, T., Stolić, I., Krstulović, L., Bajić, M., and Glavaš-Obrovac, L., J. Mol. Struct., 2017, vol. 1133, p. 66. https://doi.org/10.1016/j.molstruc.2016.11.074
Rui, M., Rossi, D., Marra, A., Paolillo, M., Schinelli, S., Curti, D., Tesei, A., Cortesi, M., Zamagni, A., Laurini, E., Pricl, S., Schepmann, D., Wűnsch, B., Urban, E., Pace, V., and Collina, S., Eur. J. Med. Chem., 2016, vol. 124, p. 649. https://doi.org/10.1016/j.ejmech.2016.08.067
Hsieh, C.Y., Ko, P.W., Chang, Y.J., Kapoor, M., Liang, Y.C., Chu, H.L., Lin, H.H., Horng, J.C., and Hsu, M.H., Molecules, 2019, vol. 24, p. 3259. https://doi.org/10.3390/molecules24183259
Lemmon, M.A. and Schlessinger, J., Cell, 2010, vol. 25, p. 1117. https://doi.org/10.1016/j.cell.2010.06.011
Kim, M., Baek, M., and Kim, D.J., Curr. Pharm. Des., 2017, vol. 23, p. 4226. https://doi.org/10.2174/1381612823666170616082125
Wang, M., Xu, S., Lei, H., Wang, C., Xiao, Z., Jia, S., Zhi, J., Zheng, P., and Zhu, W., Bioorg. Med. Chem., 2017, vol. 25, p. 5754. https://doi.org/10.1016/j.bmc.2017.09.003
Tugues, S., Koch, S., Gualandi, L., Li, X., Claesson-Welsh, L., Mol. Aspects Med., 2011, vol. 32, p. 88. https://doi.org/10.1016/j.mam.2011.04.004
El-Helby, A.G.A., Sakr, H., Eissa, I.H., Abulkhair, H., Al-Karmalawy, A.A., and El-Adl, K., Arch. Pharm. (Weinheim), 2019, vol. 352, article ID 1900113. https://doi.org/10.1002/ardp.201900113
Sharma, N., Sharma, M., Rahman, Q.I., Akhtar, S., and Muddassir, M., J. Biomol. Struct. Dyn., 2021, vol. 39, p. 2806. https://doi.org/10.1080/07391102.2020.1754916
Benassi, A., Doria, F., and Pirota, V., Int. J. Mol. Sci., 2020, vol. 21, article no. 8692. https://doi.org/10.3390/ijms21228692
Çevik, U.A., Sağlık, B.N., Ardıç, C.M., Özkay, Y., and Atlı, O., Turk. J. Biochem., 2018, vol. 43, p. 151. https://doi.org/10.1515/tjb-2017-0167
Helwa, A.A., El-Dydamony, N.M., Radwan, R.A., Abdelraouf, S.M., and Abdelnaby, R.M., Bioorg. Chem., 2020, vol. 102, article ID 104051. https://doi.org/10.1016/j.bioorg.2020.104051
Wu, L.T., Jiang, Z., Shen, J.J., Yi, H., Zhan, Y.C., Sha, M.Q., Wang, Z., Xue, S.T., Li, Z.R., Eur. J. Med. Chem., 2016, vol. 114, p. 328. https://doi.org/10.1016/j.ejmech.2016.03.029
Morais, G.R., Palma, E., Marques, F., Gano, L., Oliveira, M.C., Abrunhosa, A., Miranda, H.V., Outeiro, T.F., Santos, I., and Paulo, A., J. Heterocycl. Chem., 2017, vol. 54, p. 255. https://doi.org/10.1002/jhet.2575
Onnis, V., Demurtas, M., Deplano, A., Balboni, G., Baldisserotto, A., Manfredini, S., Pacifico, S., Liekens, S., and Balzarini, J., Molecules, 2016, vol. 21, article no. 579. https://doi.org/10.3390/molecules21050579
Root, W.J., Hiemstra, H., and Speckamp, W.N., J. Org. Chem., 1992, vol. 57, p. 1059. https://doi.org/10.1021/jo00030a002
Ahmad, A., Husain, A., Khan, S.A., Mujeeb, M., and Bhandari, A., J. Saudi Chem. Soc., 2015, vol. 19, p. 340. https://doi.org/10.1016/j.jscs.2014.05.007
Du, X., Yin, D., Ge, Z., Wang, X., and Li, R., RSC Adv., 2017, vol. 7, p. 24547. https://doi.org/10.1039/c7ra03069j
Rashid, M., Husain, A., Mishra, R., Karim, S., Khan, S., Ahmad, M., Al-wabel, N., Husain, A., Ahmad, A., and Khan, S.A., Arab. J. Chem., 2019, vol. 12, p. 3202. https://doi.org/10.1016/j.arabjc.2015.08.019
Husain, A., Rashid, M., Shaharyar, M., Siddiqui, A.A., and Mishra, R., Eur. J. Med. Chem., 2013, vol. 62, p. 785. https://doi.org/10.1016/j.ejmech.2012.07.011
Rashid, M., Husain, A., Shaharyar, M., and Sarafroz, M., Anti-Cancer Agents Med. Chem., 2014, vol. 14, p. 1003. https://doi.org/10.2174/1871520614666140509153021
Husain, A., Bhutani, M., Parveen, S., Khan, S.A., Ahmad, A., and Iqbal, M.A., J. Chin. Chem. Soc., 2021, vol. 68, p. 362. https://doi.org/10.1002/jccs.202000130
Liew, S.K., Malagobadan, S., Arshad, N.M., and Nagoor, N.H., Biomolecules, 2020, vol. 10, article no. 138. https://doi.org/10.3390/biom10010138
Reddy, T.S., Reddy, V.G., Kulhari, H., Shukla, R., Kamal, A., and Bansal, V., Eur. J. Med. Chem., 2016, vol. 117, p. 157. https://doi.org/10.1016/j.ejmech.2016.03.051
Lu, W., Li, P., Shan, Y., Su, P., Wang, J., Shi, Y., and Zhang, J., Bioorg. Med. Chem., 2015, vol. 23, p. 1044. https://doi.org/10.1016/j.bmc.2015.01.006
Wang, W., Wu, C., Wang, J., Luo, R., Wang, C., Liu, X., Li, J., Zhu, W., and Zheng, P., Bioorg. Med. Chem., 2016, vol. 24, p. 6166. https://doi.org/10.1016/j.bmc.2016.09.021
Alnoman, R.B., Hagar, M., Parveen, S., Ahmed, H.A., and Knight, J.G., J. Photochem. Photobiol., A, 2020, vol. 395, article ID 112508. https://doi.org/10.1016/j.jphotochem.2020.112508
Parveen, S., Arjmand, F., and Mohapatra, D.K., J. Photochem. Photobiol., B, 2013, vol. 126, p. 78. https://doi.org/10.1016/j.jphotobiol.2013.07.009
Parveen, S., Chem. Pap., 2021, vol. 75, p. 2339. https://doi.org/10.1007/s11696-020-01496-5
Parveen, S., Arjmand, F., and Tabassum, S., RSC Adv., 2019, vol. 9, p. 24699. https://doi.org/10.1039/c9ra04358f
Hagar, M., Chaieb, K., Parveen, S., Ahmed, H.A., and Alnoman, R.B., J. Mol. Struct., 2020, vol. 1199, article ID 126926. https://doi.org/10.1016/j.molstruc.2019.126926
Ferrara, N. and Adamis, A.P., Nat. Rev. Drug Discovery, 2016, vol. 15, p. 385. https://doi.org/10.1038/nrd.2015.17
Fedorov, O., Müller, S., and Knapp, S., Nat. Chem. Biol., 2010, vol. 6, p. 166. https://doi.org/10.1038/nchembio.297
Carmeliet, P., Nat. Med., 2003, vol. 9, p. 653. https://doi.org/10.1038/nm0603-653
Zhang, H.Q., Gong, F.H., Li, C.G., Zhang, C., Wang, Y.J., Xu, Y.G., and Sun, L.P., Eur. J. Med. Chem., 2016, vol. 109, p. 371. https://doi.org/10.1016/j.ejmech.2015.12.032
Lintnerová, L., García-Caballero, M., Gregáň, F., Melicherčík, M., Quesada, A.R., Dobiaš, J., Lác, J., Sališová, M., and Boháč, A., Eur. J. Med. Chem., 2014, vol. 72, p. 146. https://doi.org/10.1016/j.ejmech.2013.11.023
Zuccotto, F., Ardini, E., Casale, E., and Angiolini, M., J. Med. Chem., 2010, vol. 53, p. 2681. https://doi.org/10.1021/jm901443h
Schrödinger Release 2017-4: Maestro, Schrödinger, LLC, New York NY, 2017.
Banks, W.A., BMC Neurol., 2009, vol. 9, p. S3. https://doi.org/10.1186/1471-2377-9-S1-S3
ACKNOWLEDGMENTS
S. Parveen is grateful to Jamia Hamdard for “JH Silver Jubilee Post-Doctoral Fellowship-2016.”
Author information
Authors and Affiliations
Contributions
Conceptualization: A. Husain; methodology: A. Husain, M. Bhutani, S.A. Khan; formal analysis and investigation: S. Parveen, M. Bhutani, A. Iqbal, A. Ahmad; original draft preparation: S. Parveen, A. Husain; review and editing: S.A. Khan, A. Ahmad; resources: A. Husain; supervision: A. Husain.
Corresponding author
Ethics declarations
The authors confirm that this article content has no conflict of interest.
Rights and permissions
About this article
Cite this article
Husain, A., Bhutani, M., Parveen, S. et al. Design, Synthesis, In Vitro Cytotoxicity, ADME Prediction, and Molecular Docking Study of Benzimidazole-Linked Pyrrolone and N-Benzylpyrrolone Derivatives. Russ J Org Chem 58, 1438–1450 (2022). https://doi.org/10.1134/S1070428022100098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022100098