Abstract
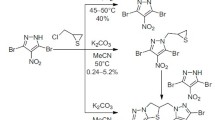
Reactions of 2-(chloromethyl)thiirane with symmetrically substituted C-bromo/nitropyrazoles in water in the presence of bases were accompanied by thiirane–thietane rearrangement to afford 4-bromo(nitro)- and 3,5-dibromo-4-bromo(nitro)-1-(thietan-3-yl)-1H-pyrazoles as convenient intermediate products for further transformations. Possible modifications of the title compounds via oxidation to 1-(1-oxo-λ4-thietan-3-yl)- and 1-(1,1-dioxo-λ6-thietan-3-yl)pyrazoles, reactions with oxygen and nitrogen nucleophiles with the formation of thietane-containing 5-methoxy- and 5-(morpholin-4-yl)-1H-pyrazoles, and reduction to 4-amino-3-bromo-5-(morpholin-4-yl)-1-(thietan-3-yl)-1H-pyrazole have been demonstrated.





Similar content being viewed by others
REFERENCES
Khaliullin, F.A., Klen, E.E., Makarova, N.N., Shepilova, S.O., and Baikova, I.P., Chem. Heterocycl. Compd., 2020, vol. 56, p. 1213. https://doi.org/10.1007/s10593-020-02800-7
Alanzy, A., Bakhotm, D., and Abdel-Rahman, R., Int. J. Org. Chem., 2020, vol. 10, p. 39. https://doi.org/10.4236/ijoc.2020.102003
Bratenko, M.K., Chornous, V.A., Voloshin, N.P., and Vovk, M.V., Chem. Heterocycl. Compd., 1999, vol. 35, p. 1075. https://doi.org/10.1007/BF02251799
Neunhoeffer, H., Gerstung, S., Clausen, Th., and Balzer, W.R., US Patent no. 5534267A, 1996.
Fessmann, Th. And Terranova, E., EP Patent Appl. no. 1406874A1, 2004.
Dahlgren, R.M., Laidig, W.D., Lim Mu-ill, Murphy, P.M., and Zhang, G., US Patent no. 7850742, 2010.
Lopyrev, V.A., Elokhina, V.N., Krylova, O.V., Nakhmanovich, A.S., Larina, L.I., Sorokin, M.S., and Vokin, A.I., Chem. Heterocycl. Compd., 1999, vol. 35, p. 1109. https://doi.org/10.1007/BF02251807
Elokhina, V.N., Krylova, O.V., Larina, L.I., Nakhmanovich, A.S., Sorokin, M.S., Volkova, K.A., and Lopyrev, V.A., Chem. Heterocycl. Compd., 2000, vol. 36, p. 476. https://doi.org/10.1007/bf02269549
Khan, K.M., Maharvi, G.M., Khan, M.T.H., Perveen, Sh., Choudhary, M.I., and Atta-ur-Rahman, Mol. Diversity, 2005, vol. 9, p. 15. https://doi.org/10.1007/s11030-005-1299-5
Usami, Y., Tatsui, Y., Yoneyama, H., and Harusawa, S., Molecules, 2020, vol. 25, article no. 4634. https://doi.org/10.3390/molecules25204634
Brahim, M., Ben Ammar, H., Soulé, J.-Fr., and Doucet, H., Tetrahedron Lett., 2016, vol. 72, p. 4312. https://doi.org/10.1016/j.tet.2016.05.079
Iaroshenko, V.O., Gevorgyan, A., Davydova, O., Villinger, A., and Langer, P., J. Org. Chem., 2014, vol. 79, p. 2906. https://doi.org/10.1021/jo4025418
Khera, R.Ah., Ali, A., Rafique, H., Hussain, M., Tatar, J., Saed, A., Villinger, A., and Langer, P., Tetrahedron Lett., 2011, vol. 67, p. 5244. https://doi.org/10.1016/j.tet.2011.05.036
Iddon, B., Tønder, J.E., Hosseini, M., and Begtrup, M., Tetrahedron, 2007, vol. 63, p. 56. https://doi.org/10.1016/j.tet.2006.10.009
D’yachenko, V.S., Danilov, D.V., Shkineva, T.K., Vatsadze, I.A., Burmistrov, V.V., and Butov, G.M., Chem. Heterocycl. Compd., 2019, vol. 55, p. 129. https://doi.org/10.1007/s10593-019-02428-2
Zabierek, A.A., Konrad, K.M., and Haidle, A.M., Tetrahedron Lett., 2008, vol. 49, p. 2996. https://doi.org/10.1016/j.tetlet.2008.02.169
Estrada, A.A., Feng, J.A., Lyssikatos, J.P., Sweeney, Z.K., and De Vicente Fidalgo, J., Int. Patent Appl. Pub. no. WO2017218843, 2017.
Osyanin, V.A., Nakushnov, V.Y., and Klimochkin, Y.N., Chem. Heterocycl. Compd., 2011, vol. 47, p. 755. https://doi.org/10.1007/s10593-011-0830-0
Osipov, D.V., Osyanin, V.A., Voskressensky, L.G., and Klimochkin, Y.N., Synthesis, 2017, vol. 49, p. 2286. https://doi.org/10.1055/s-0036-1588411
Pharmaceutical Substances—Online Edition (Version 4.9), Kleemann, A., Engel, J., Kutscher, B., and Reichert, D., Eds., Stuttgart: Thieme. 2020.
Teegarden, B.R., Li, H., Jayakumar, H., StrahPleynet, S., Dosa, P.I., Selaya, S.D., Kato, N., Elwell, K.H., Davidson, J., Cheng, K., Saldana, H., Frazer, J.M., Whelan, K., Foster, J., Espitia, S., Webb, R.R., Beeley, N.R.A., Thomsen, W., Morairty, S.R., Kilduff, Th.S., and Al-Shamma, H.A., J. Med. Chem., 2010, vol. 53, p. 1923. https://doi.org/10.1021/jm9007328
Dalinger, I.L., Kormanov, A.V., Vatsadze, I.A., Shkineva, T.K., Kozeev, A.M., Averkiev, B.B., and Sheremetev, A.B., Chem. Heterocycl. Compd., 2015, vol. 51, p. 819. https://doi.org/10.1007/s10593-015-1781-7
Xu, D., Frank, L., Nguyen, T., Stumpf, A., Russell, D., Angelaud, R., and Gosselin, F., Synlett, 2020, vol. 31, p. 595. https://doi.org/10.1055/s-0039-1690160
Petko, K.I., Sokolenko, T.M., Filatov, A.A., Polovinko, V.V., Rusanov, E.B., Dudko, V.A., and Yagupolskii, Y.L., Chem. Heterocycl. Compd., 2019, vol. 55, p. 359. https://doi.org/10.1007/s10593-019-02465-x
Nicolaou, K.C., Rhoades, D., Wang, Y., Totokotsopoulos, S., Bai, R., and Hamel, E., ChemMedChem, 2015, vol. 10, p. 1974. https://doi.org/10.1002/cmdc.201500401
Zaitsev, A.A., Vatsadze, I.A., Dalinger, I.L., Kachala, V.V., Nelyubina, Yu.V., and Shevelev, S.A., Russ. Chem. Bull., Int. Ed., 2009, vol. 58, p. 2109. https://doi.org/10.1007/s11172-009-0288-8
Leśniak, S., Kinart, W.J., and Lewkowski, J., Comprehensive Heterocyclic Chemistry III, Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., and Taylor, R.J.K., Eds., Amsterdam: Elsevier, 2008, vol. 2, p. 389. https://doi.org/10.1016/B978-008044992-0.00207-8
Gurevich, K.G., Urakov, A.L., Klen, E.E., Samorodov, A.V., Nikitina, I.L., Khaliullin, F.A., Nebogatova, V.A., Makarova, N.N., Shepilova, S.O., Bashirova, L.I., and Khalimov, A.R., Pharm. Chem. J., 2020, vol. 54, p. 213. https://doi.org/10.1007/s11094-020-02182-2
Klen, E.E. and Khaliullin, F.A., Russ. J. Org. Chem., 2009, vol. 45, p. 135. https://doi.org/10.1134/S1070428009010187
Contreras, J.G., Hurtado, M.S., Gerli, L.A., and Madariaga, S.T., J. Mol. Struct.: THEOCHEM, 2005, vol. 713, p. 207. https://doi.org/10.1016/j.theochem.2004.10.014
Dittmer, D.C., Patwardhan, B.H., and Bartholomew, J.T., Org. Magn. Reson., 1982, vol. 18, p. 82. https://doi.org/10.1002/mrc.1270180207
ACKNOWLEDGMENTS
The NMR spectra were recorded using the facilities of the Chemistry joint center (Ufa Institute of Chemistry, Ufa Federal Research Center, Russian Academy of Sciences) and Agidel regional joint center (Ufa Federal Research Center, Russian Academy of Sciences).
Funding
This study was performed in the framework of state assignment no. AAAA-A20-120012090029-0.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2022, Vol. 58, No. 9, pp. 926–935 https://doi.org/10.31857/S0514749222090026.
For communication I, see [1].
Rights and permissions
About this article
Cite this article
Klen, E.E., Makarova, N.N., Khaliullin, F.A. et al. Reactions of Thiiranes with NH Heterocycles: II. C-Bromo/Nitro-1-(thietan-3-yl)pyrazoles as Convenient Synthons for Substituted 1-(Thietan-3-yl)pyrazoles. Russ J Org Chem 58, 1192–1199 (2022). https://doi.org/10.1134/S1070428022090020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022090020