Abstract
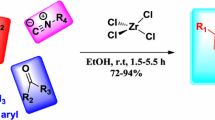
An efficient one-pot synthesis of hexahydroquinoline derivatives by the condensation of aromatic aldehydes, 5,5-dimethylcyclohexane-1,3-dione, ethyl (or methyl) acetoacetate, and ammonium acetate in the presence of zirconium(IV) oxychloride octahydrate (ZrOCl2·8H2O) as catalyst has been developed. The reaction was performed under conventional heating, as well as under microwave irradiation. The developed methodology (microwave-assisted method) offers several advantages, including shorter reaction time, milder conditions, easy work-up, inexpensive catalyst, and excellent yields. The structure of the synthesized compounds has been established on the basis of their spectral and analytical data.

Similar content being viewed by others
REFERENCES
Gawande, M.B., Rathi, A.K., Tucek, J., Safarova, K., Bundaleski, N., Teodoro, O.M.N.D., Kvitek, L., Varma, R.S., and Zboril, R., Green Chem., 2014, vol. 16, p. 4137. https://doi.org/10.1039/c4gc00774c
Lidström, P., Tierney, J., Wathey, B., and Westman, J., Tetrahedron, 2001, vol. 57, p. 9225. https://doi.org/10.1016/S0040-4020(01)00906-1
Ravichandran, S. and Karthikeyan, E., Int. J. ChemTech Res., 2011, vol. 3, p. 466.
Chetan, K.J., Amol, S.N., Asha, V.C., Vishal, D.S., Anil, P.P., and Charansingh, H.G., ACS Omega, 2020, vol. 5, p. 29055. https://doi.org/10.1021/acsomega.0c03575
Shaabani, S., Shaabani, A., and Ng, S.W., ACS Comb. Sci., 2014, vol. 16, p. 176. https://doi.org/10.1021/co4001259
Banerjee, S. and Saha, A., New J. Chem., 2013, vol. 37, p. 4170. https://doi.org/10.1039/c3nj00723e
Xin, J., Chang, L., Hou, Z., Shang, D., Liu, X., and Feng, X., Chem. Eur. J., 2008, vol. 14, p. 3177. https://doi.org/10.1002/chem.200701581
Marella, A., Tanwar, O.P., Saha, R., Ali, M.R., Srivastava, S., Akhter, M., Shaquiquzzaman, M., and Mumtaz Alam, M., Saudi Pharm. J., 2013, vol. 21, p. 1. https://doi.org/10.1016/j.jsps.2012.03.002
Prescott, T.A.K., Sadler, I.H., Kiapranis, R., and Maciver, S.K., J. Ethnopharmacol., 2007, vol. 109, p. 289. https://doi.org/10.1016/j.jep.2006.07.036
Bridges, A.J., Moos, W.H., Szotek, D.L., Trivedi, B.K., Bristol, J.A., Heffner, T.G., Bruns, R.F., and Downs, D.A., J. Med. Chem., 1987, vol. 30, p. 1709. https://doi.org/10.1021/jm00393a003
Jarak, I., Kralj, M., Piantanida, I., Šuman, L., Žinić, M., Pavelić, K., and Karminski-Zamola, G., Bioorg. Med. Chem., 2006, vol. 14, p. 2859. https://doi.org/10.1016/j.bmc.2005.12.004
Tasqeeruddin, S., Yahya, I.A., and Jaber, A.A., Lett. Org. Chem., 2020, vol. 17, p. 157. https://doi.org/10.2174/1570178616666190618153721
Tasqeeruddin, S. and Yahya, I.A., J. Heterocycl. Chem., 2020, vol. 57, p. 132. https://doi.org/10.1002/jhet.3754
Fehnel, E.A., J. Org. Chem., 1966, vol. 31, p. 2899. https://doi.org/10.1021/jo01347a038
Yadav, G.D., Kumbhar, R.P., and Helder, S., Int. Rev. Chem. Eng., 2012, vol. 4, p. 597.
Nelson, J.L. and Kermit, J.C., J. Heterocycl. Chem., 1966, vol. 3, p. 485. https://doi.org/10.1002/jhet.5570030420
Pfitzinger, W., J. Prakt. Chem., 1885, vol. 33, p. 100. https://doi.org/10.1002/prac.18850330110
Yadav, J.S., Rao, P.P., Sreenu, D., Rao, R.S., Kumar, V.N., Nagaiah, K., and Prasad, A.R., Tetrahedron Lett., 2005, vol. 46, p. 7249. https://doi.org/10.1016/j.tetlet.2005.08.042
Wu, J., Xia, H.-G., and Gao, K., Org. Biomol. Chem., 2006, vol. 4, p. 126. https://doi.org/10.1039/B514635F
Arcadi, A., Chiarini, M., Di Giuseppe, S., and Marinelli, F., Synlett, 2003, vol. 2003, no. 2, p. 203. https://doi.org/10.1055/s-2003-36798
Song, S.J., Cho, S.J., Park, D.K., Kwon, T.W., and Jenekhe, S.A., Tetrahedron Lett., 2003, vol. 44, p. 255. https://doi.org/10.1016/S0040-4039(02)02499-1
Palimkar, S.S., Siddiqui, S.A., Daniel, T., Lahoti, R.J., and Srinivasan, K.V., J. Org. Chem., 2003, vol. 68, p. 9371. https://doi.org/10.1021/jo035153u
Swenson, R.E., Sowin, T.J., and Zhang, H.Q., J. Org. Chem., 2002, vol. 67, p. 9182. https://doi.org/10.1021/jo0203387
Cho, C.K., Hooh, B., and Shim, S.C., J. Heterocycl. Chem., 1999, vol. 36, p. 1175. https://doi.org/10.1002/jhet.5570360510
Shaik, M.G., Yadavalli, S.K., Jong, S.J., Jong, P.K., Jong, S.B., Eun, H.C., Do, Y.K., Eun, K.J., Fazlur-Rahman, N.K., and Euh, D.J., RSC Adv., 2014, vol. 4, p. 44408. https://doi.org/10.1039/c4ra06772j
Sangu, K., Fuchibe, K., and Akiyama, T., Org. Lett., 2004, vol. 6, p. 353. https://doi.org/10.1021/ol036190a
Crousse, B., Bégué, J.-P., and Bonnet-Delpon, D., Tetrahedron Lett., 1998, vol. 39, p. 5765. https://doi.org/10.1016/S0040-4039(98)01202-7
Crousse, B., Bégué, J.-P., and Bonnet-Delpon, D., J. Org. Chem., 2000, vol. 65, p. 5009. https://doi.org/10.1021/jo9918807
Banwell, M.G., Lupton, D.W., Ma, X., Renner, J., and Sydnes, M.O., J. Org. Chem., 2004, vol. 6, p. 2741. https://doi.org/10.1021/ol0490375
Shengkai, K., Sastry, M.N.V., Chunchi, L., and Ching-Fa, Y., Tetrahedron Lett., 2005, vol. 46, p. 5771. https://doi.org/10.1016/j.tetlet.2005.05.148
James, L.D., Richard, A.G., and Surya, K.D., J. Mol. Catal. A: Chem., 2006, vol. 256, p. 309. https://doi.org/10.1016/j.molcata.2006.03.079
ACKNOWLEDGMENTS
The authors are thankful to Dean of Scientific Research, King Khalid University, and the College of Pharmacy, Department of Pharmaceutical Chemistry, for providing facilities to carry out our research work.
Funding
This work was supported by the Dean of Scientific Research, King Khalid University, according to the General Research Project, grant no. R.G.P2/83/41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Tasqeeruddin, S., Asiri, Y.I. & Shaheen, S. Zirconium(IV) Oxychloride Octahydrate (ZrOCl2·8H2O): An Efficient Catalyst for the One-Pot Multicomponent Synthesis of Hexahydroquinoline Derivatives under Conventional Heating and Microwave Irradiation. Russ J Org Chem 58, 1008–1014 (2022). https://doi.org/10.1134/S1070428022070107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022070107