Abstract
The condensation of propargylated vanillin with differently substituted acetophenones produced the corresponding chalcones which were reacted with substituted benzyl azides using the click chemistry technique to afford triazole–chalcone hybrids in 34–93% yields. The synthesized hybrid compounds were evaluated for their antitubercular activity, and the results showed synergistic effect of the triazole and chalcone pharmacophores combined in a single molecule. The most potent compounds were characterized by a MIC value of 1.6 μg/mL. Molecular docking studies of the most active compounds against the mycobacterial protein PDB 4Y6U were performed.


Similar content being viewed by others
REFERENCES
Chakraborty, S. and Rhee, K.Y., Cold Spring Harbor Perspect. Med., 2015, vol. 5, article ID 021147. https://doi.org/10.1101/cshperspect.a021147
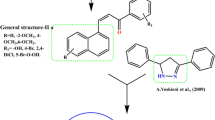
Viegas-Junior, C., Danuello, A., da Silva Bolzani, V., Barreiro, E.J., and Fraga, C.A.M., Curr. Med. Chem., 2007, vol. 14, p. 1829. https://doi.org/10.2174/092986707781058805
Bérubé, G., Expert Opin. Drug Discovery, 2016, vol. 11, p. 281. https://doi.org/10.1517/17460441.2016.1135125
Boshra, A.N., Abdu-Allah, H.H., Mohammed, A.F., and Hayallah, A.M., Bioorg. Chem., 2020, vol. 95, article ID 103505. https://doi.org/10.1016/j.bioorg.2019.103505
Manna, T., Pal, K., Jana, K., and Misra, A.K., Bioorg. Med. Chem. Lett., 2019, vol. 29, article ID 126615. https://doi.org/10.1016/j.bmcl.2019.08.019
Rezki, N., Mayaba, M.M., Al-blewi, F.F., Aouad, M.R., and El-Ashry, E.S.H., Res. Chem. Intermed., 2017, vol. 43, p. 995. https://doi.org/10.1007/s11164-016-2679-4
Lakkakula, R., Roy, A., Mukkanti, K., and Sridhar, G., Russ. J. Gen. Chem., 2019, vol. 89, p. 831. https://doi.org/10.1134/S1070363219040315
Raju, K.S., AnkiReddy, S., Sabitha, G., Krishna, V.S., Sriram, D., Reddy, K.B., and Sagurthi, S.R., Bioorg. Med. Chem. Lett., 2019, vol. 29, no. 2, p. 284. https://doi.org/10.1016/j.bmcl.2018.11.036
McFadden, K., Fletcher, P., Rossi, F., Umashankara, M., Pirrone, V., Rajagopal, S., Gopi, H., Krebs, F.C., Martin-Garcia, J., Shattock, R.J., and Chaiken, I., Antimicrob. Agents Chemother., 2012, vol. 56, p. 1073. https://doi.org/10.1128/AAC.05555-11
Kim, T.W., Yong, Y., Shin, S.Y., Jung, H., Park, K.H., Lee, Y.H., Lim, Y., and Jung, K.Y., Bioorg. Chem., 2015, vol. 59, p. 1. https://doi.org/10.1016/j.bioorg.2015.01.003
Devender, N., Gunjan, S., Chhabra, S., Singh, K., Pasam, V.R., Shukla, S.K., Sharma, A., Jaiswal, S., Singh, S.K., Kumar, Y., and Lal, J., Eur. J. Med. Chem., 2016, vol. 109, p. 187. https://doi.org/10.1016/j.ejmech.2015.12.038
Sahu, J.K., Ganguly, S., and Kaushik, A., Chin. J. Nat. Med., 2013, vol. 11, p. 456. https://doi.org/10.1016/S1875-5364(13)60084-9
Kumar, S.K., Hager, E., Pettit, C., Gurulingappa, H., Davidson, N.E., and Khan, S.R., J. Med. Chem., 2003, vol. 46, p. 2813. https://doi.org/10.1021/jm030213+
Gomes, M.N., Braga, R.C., Grzelak, E.M., Neves, B.J., Muratov, E., Ma, R., and Andrade, C.H., Eur. J. Med. Chem., 2017, vol. 137, p. 126. https://doi.org/10.1016/j.ejmech.2017.05.026
Anand, N., Singh, P., Sharma, A., Tiwari, S., Singh, V., Singh, D.K., and Tripathi, R.P., Bioorg. Med. Chem., 2012, vol. 20, p. 5150. https://doi.org/10.1016/j.bmc.2012.07.009
Lahtchev, K.L., Batovska, D.I., Parushev, St.P., Ubiyvovk, V.M., and Sibirny, A.A., Eur. J. Med. Chem., 2008, vol. 43, p. 2220. https://doi.org/10.1016/j.ejmech.2007.12.027
Kant, R., Kumar, D., Agarwal, D., Gupta, R.D., Tilak, R., Awasthi, S.K., and Agarwal, A., Eur. J. Med. Chem., 2016, vol. 113, p. 34. https://doi.org/10.1016/j.ejmech.2016.02.041
Dan, W. and Dai, J., Eur. J. Med. Chem., 2020, vol. 187, article ID 111980. https://doi.org/10.1016/j.ejmech.2019.111980
Nowakowska, Z., Eur. J. Med. Chem., 2007, vol. 42, p. 125. https://doi.org/10.1016/j.ejmech.2006.09.019
Vlachakis, D.P., Molecular Docking, Vlachakis, D.P., Ed., London: IntechOpen, 2018, chap. 1, p. 3. https://doi.org/10.5772/intechopen.78266
Huang, B., OMICS: J. Integr. Biol., 2009, vol. 13, p. 325. https://doi.org/10.1089/omi.2009.0045
Phillips, M.A., Stewart, M.A., Woodling, D.L. and Xie, Z.R., Molecular Docking, Vlachakis, D.P., Ed., London: IntechOpen, 2018, chap. 8, p. 141. https://doi.org/10.5772/intechopen.72898
Swietnicki, W. and Brzozowska, E., J. Mol. Graphics Modell., 2019, vol. 92, p. 8. https://doi.org/10.1016/j.jmgm.2019.07.002
Azam, S.S. and Abbasi, S.W., Theor. Biol. Med. Modell., 2013, vol. 10, article no. 63. https://doi.org/10.1186/1742-4682-10-63
Jayamani, A., Sengottuvelan, N., Kang, S.K., and Kim, Y.I., Inorg. Chem. Commun., 2014, vol. 48, p. 147. https://doi.org/10.1016/j.inoche.2014.08.029
Amirmostofian, M., Kobarfard, F., Reihanfard, H., Mashayekhi, V., and Zarghi, A., Iran. J. Pharm. Res., 2015, vol. 14, p. 59.
Lourenco, M.C.S., de Souza, M.V.N., Pinheiro, A.C., de Ferreira, L.M., Goncalves, R.S.B., Nogueira, T.C.M., and Peralta, M.A., Arkivoc, 2007, vol. 2007, part (xv), p. 181. https://doi.org/10.3998/ark.5550190.0008.f18
Albesa-Jové, D., Mendoza, F., Rodrigo-Unzueta, A., Gomollón-Bel, F., Cifuente, J.O., Urresti, S., Comino, N., Gómez, H., Romero-García, J., Lluch, J.M., Sancho-Vaello, E., Biarnés, X., Planas, A., Merino, P., Masgrau, L., and Guerin, M.E., Angew. Chem., Int. Ed., 2015, vol. 54, p. 9898. https://doi.org/10.1002/anie.201504617; ProteinDataBank. https://doi.org/10.2210/pdb4Y6U/pdb
Trott, O. and Olson, A.J., J. Comput. Chem., 2010, vol. 31, p. 455. https://doi.org/10.1002/jcc.21334
Seeliger, D. and Groot, B.L., J. Comput.-Aided Mol. Des., 2010, vol. 24, p. 417. https://doi.org/10.1007/s10822-010-9352-6
Lill, M.A. and Danielson, M.L., J. Comput.-Aided. Mol. Des., 2011, vol. 25, p. 13. https://doi.org/10.1007/s10822-010-9395-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Kaur, H., Singh, R. & Rishikant Synthesis, Molecular Docking, and Antitubercular Evaluation of Triazole–Chalcone Conjugates. Russ J Org Chem 58, 518–525 (2022). https://doi.org/10.1134/S107042802204008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042802204008X