Abstract
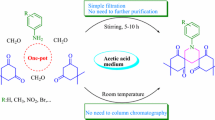
A one-pot procedure has been developed for the synthesis of 2,9-bis(halophenyl)-substituted (3bR*,7aR*,10bR*,14aR*)-octadecahydro-1H,8H-2,3a,7b,9,10a,14b-hexaazadibenzo[fg,op]tetracenes by multicomponent condensation of trans-1,6,7,12-tetraazaperhydrotetracene with formaldehyde and haloanilines in the presence of YbCl3·6H2O as catalyst.

Similar content being viewed by others
REFERENCES
Neumann, P., Aumueller, A., and Trauth, H., US Patent no. 4904779, 1990.
Rakhimova, E.B., Kirsanov, V.Yu., Mescheryakova, E.S., Khalilov, L.M., Ibragimov, A.G., and Dzhemilev, U.M., Synlett, 2018, vol. 29, p. 1861. https://doi.org/10.1055/s-0037-1610201
Rakhimova, E.B., Kirsanov, V.Yu., Mescheryakova, E.S., Ibragimov, A.G., and Dzhemilev, U.M., Mendeleev Commun., 2020, vol. 30, p. 308. https://doi.org/10.1016/j.mencom.2020.05.015
Rakhimova, E.B., Kirsanov, V.Yu., Tret’yakova, E.V., Khalilov, L.M., Ibragimov, A.G., Dzhemileva, L.U., D’yakonov, V.A., and Dzhemilev, U.M., RSC Adv., 2020, vol. 10, p. 21039. https://doi.org/10.1039/D0RA03209C
Dragoun, M., Gunther, T., Frias, C., Berkessel, A., and Prokop, A., J. Cancer Res. Clin. Oncol., 2018, vol. 144, p. 685. https://doi.org/10.1007/s00432-018-2592-x
Hopff, S.M., Wang, Q., Frias, C., Ahrweiler, M., Wilke, N., Wilke, N., Berkessel, A., and Prokop, A., J. Cancer Res. Clin. Oncol., 2021, vol. 147, p. 2591. https://doi.org/10.1007/s00432-021-03679-3
Khokhar, A.R., Al-Baker, S., Shamsuddin, S., and Siddik, Z.H., J. Med. Chem., 1997, vol. 40, p. 112. https://doi.org/10.1021/jm960587l
Morales, F., Ramirez, A., Morata-Tarifa, C., Navarro, S.A., Marchal, J.A., Campos, J.M., and Conejo-Garcia, A., Future Med. Chem., 2017, vol. 9, p. 293. https://doi.org/10.4155/fmc-2016-0212
Omer, K.H., Seliman, A.A., Altaf, M., Casagrande, N., Aldinucci, D., Altuwaijri, S., and Isab, A.A., Polyhedron, 2015, vol. 102, p. 773. https://doi.org/10.1016/j.poly.2015.10.029
Iwanejko, J., Wojaczynska, E., Trynda, J., Maciejewska, M., Wietrzyk, J., Kochel, A., and Wojaczynski, J., Tetrahedron, 2017, vol. 73, p. 2276. https://doi.org/10.1016/j.tet.2017.03.017
Rakhimova, E.B., Kirsanov, V.Yu., Ibragimov, A.G., and Dzhemilev, U.M., Russ. J. Org. Chem., 2018, vol. 54, p. 1085. https://doi.org/10.1134/S1070428018070199
Barsegyan, Y.A., Baranov, V.V., Kravchenko, A.N., Strelenko, Y.A., Anikina, L.V., Karnoukhova, V.A., and Kolotyrkina, N.G., Synthesis, 2018, vol. 50, p. 2099. https://doi.org/10.1055/s-0036-1591952
ACKNOWLEDGMENTS
The spectral studies were performed using the facilities of the Agidel joint center (Institute of Petrochemistry and Catalysis, Ufa Federal Research Center, Russian Academy of Sciences).
Funding
This study was performed according to the research program of the Institute of Petrochemistry and Catalysis (Ufa Federal Research Center, Russian Academy of Sciences), project no. FMRS-2022-0079 for 2022–2024 (Multicomponent Catalytic Reactions in the Synthesis of Cyclic and Acyclic Heteroatom Compounds). It was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Federal special-purpose program no. 2019-05-595-000-058) and by the President scholarship to young scientists and post-graduate students (project no. SP-197.2019.4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2022, Vol. 58, No. 3, pp. 311–316 https://doi.org/10.31857/S0514749222030090.
Rights and permissions
About this article
Cite this article
Rakhimova, E.B., Kirsanov, V.Y. & Ibragimov, A.G. One-Pot Synthesis of 2,9-Bis(halophenyl)-Substituted Perhydrohexaazadibenzotetracenes. Russ J Org Chem 58, 322–326 (2022). https://doi.org/10.1134/S1070428022030095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022030095