Abstract
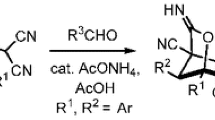
Tetracyanoethylene adducts with ketones, 4-oxoalkane-1,1,2,2-tetracarbonitriles reacted with hydrogen chloride or phosphorus(III) chloride in 1,4-dioxane to give 3-chloro-6-oxo-2,7-diazabicyclo[3.2.1]oct-3-ene-4,5-dicarbonitrile derivatives.
Similar content being viewed by others
References
Barlocco, D., Cignarella, G., Tondi, D., Vianello, P., Villa, S., Bartolini, A., Ghelardini, C., Galeotti, N., Anderson, D.J., Kuntzweiler, T.A., Colombo, D., and Toma, L., J. Med. Chem., 1998, vol. 41, p. 674. doi https://doi.org/10.1021/jm970427p
Liu, H., Cheng, T.-M., Zhang, H.-M., and Li, R.-T., Arch. Pharm., 2003, vol. 336, p. 510. doi https://doi.org/10.1002/ardp.200300749
Filosa, R., Peduto, A., de Caprariis, P., Saturnino, C., Festa, M., Petrella, A., Pau, A., Pinna, G.A., La Colla, P., Busonera, B., and Loddo, R., Eur. J. Med. Chem., 2007, vol. 42, p. 293. doi j.ejmech.2006.11.013.
Villa, S., Barlocco, D., Cignarella, G., Papp, G.P., Baláti, B., Takács, J., Varró, A., Borosy, A., Keserû, K., and Mátyus, P., Eur. J. Med. Chem., 2001, vol. 36, p. 495. doi https://doi.org/10.1016/S0223-5234(01)01246-6
Gordon, E.M., Duncton, M.A.J., and Gallop, M.A., J. Med. Chem., 2018, vol. 61, p. 10340. doi https://doi.org/10.1021/acs.jmedchem.8b01389
Dinsmore, C.J., Bergman, J.M., Bogusky, M.J., Culberson, J.C., Hamilton, K.A., and Graham, S.L., Org. Lett., 2001, vol. 3, p. 865. doi https://doi.org/10.1021/ol015504w
Kang, Z., Zhang, D., and Hu, W., Org. Lett., 2017, vol. 19, p. 3783. doi https://doi.org/10.1021/acs.orglett.7b01664
Marino, J.P., Kim, M.W., and Lawrence, R., J. Org. Chem., 1989, vol. 54, p. 1782. doi https://doi.org/10.1021/jo00269a004
Inoue, K., Ishikawa, Y., and Nishiyama, S., Org. Lett., 2010, vol. 12, p. 436. doi https://doi.org/10.1021/o1902566p
Ershov, O.V., Ievlev, M.Yu., Belikov, M.Yu., and Nasakin, O.E., Russ. J. Org. Chem., 2016, vol. 52, p. 1353. doi https://doi.org/10.1134/S1070428016090189
Ershov, O.V., Maksimova, V.N., Lipin, K.V., Belikov, M.Y., Ievlev, M.Y., Tafeenko, V.A., and Nasakin, O.E., Tetrahedron, 2015, vol. 71, p. 7445. doi https://doi.org/10.1016/j.tet.2015.06.031
Kayukov, Y.S., Lukin, P.M., Nasakin, O.E., Khrustalev, V.N., Nesterov, V.N., Antipin, M.Y., and Lyshchikov, A.N., Chem. Heterocycl. Compd., 1997, vol. 33, p. 452. doi https://doi.org/10.1007/BF02321392
Kayukov, Y.S., Lukin, P.M., Nasakin, O.E., Urman, Y.G., Khrustalev, V.N., Nesterov, V.N., Antipin, M.Y., and Sheverdov, V.V., Chem. Heterocycl. Compd., 1997, vol. 33, p. 457. doi https://doi.org/10.1007/BF02321393
Nasakin, O.E., Sheverdov, V.P., Ershov, O.V., Moiseeva, I.V., Lyshchikov, A.N., Khrustalev, V.N., and Antipin, M.Y., Mendeleev Commun, 1997, vol. 7, p. 112. doi https://doi.org/10.1070/MC1997v007n03ABEH000722
Lipin, K.V., Fedoseev, S.V., Ershov, O.V., and Tafeenko, V.A., Russ. J. Org. Chem., 2017, vol. 53, p. 1828. doi https://doi.org/10.1134/S1070428017120077
Ershov, O.V., Lipin, K.V., Eremkin, A.V., Ershov, O.V., Kayukov, Ya.S., and Nasakin, O.E., Russ. J. Org. Chem., 2009, vol. 45, p. 470. doi https://doi.org/10.1134/S1070428009030245
Eremkin, A.V., Ershov, O.V., Lipin, K.V., Kayukov, Ya.S., Nasakin, O.E., and Tafeenko, V.A., Russ. J. Org. Chem., 2009, vol. 45, p. 1541. doi https://doi.org/10.1134/S1070428009100212
Ershov, O.V., Lipin, K.V., Eremkin, A.V., Nasakin, O.E., Sheverdov, V.P., Fedorov, P.I., and Tafeenko, V.A., Russ. J. Org. Chem., 2017, vol. 53, p. 215. doi https://doi.org/10.1134/S1070428017020129
Ershov, O.V., Ievlev, M.Y., Belikov, M.Y., Lipin, K.V., Naydenova, A.I., and Tafeenko, V.A., RSC Adv., 2016, vol. 6, p. 82227. doi https://doi.org/10.1039/C6RA16787J
Lipin, K.V., Maksimova, V.N., Ershov, O.V., Eremkin, A.V., Kayukov, Ya.S., and Nasakin, O.E., Russ. J. Org. Chem., 2010, vol. 46, p. 617. doi https://doi.org/10.1134/S1070428017070120
Belikov, M.Yu., Ievlev, M.Yu., Ershov, O.V., Lipin, K.V., Legotin, S.A., and Nasakin, O.E., Russ. J. Org. Chem., 2014, vol. 50, p. 1372. doi https://doi.org/10.1134/S1070428017050086
Eremkin, A.V., Molkov, S.N., Ershov, O.V., Kayukov, Y.S., Nasakin, O.E., Tafeenko, V.A., and Nurieva, E.V., Mendeleev Commun., 2006, vol. 16, p. 115. doi https://doi.org/10.1070/MC2006v016n02ABEH002206
Ershov, O.V. and Ievlev, M.Y., Chem. Heterocycl. Compd., 2017, vol. 53, p. 948. doi https://doi.org/10.1007/s10593-017-2155-0
Funding
This study was performed in the framework of the base part of state assignment of the Ministry of Science and Higher Education of the Russian Federation [project no. 4.6283.2017/8.9 (0849-2017-0001].
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
The authors declare no conflict of interests.
Russian Text © The Author(s), 2019, published in Zhurnal Organicheskoi Khimii, 2019, Vol. 55, No. 7, pp. 1115–1119.
Rights and permissions
About this article
Cite this article
Ershov, O.V., Lipin, K.V., Belikov, M.Y. et al. Synthesis of 2,7-Diazabicyclo[3.2.1]oct-3-ene Derivatives. Russ J Org Chem 55, 1009–1012 (2019). https://doi.org/10.1134/S1070428019070170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019070170