Abstract
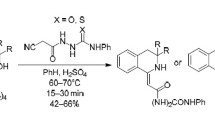
Aiming at the synthesis of new potentially pharmacologically active compounds combining in the molecule structures of thiosemicarbazone and 3-hydrazinylpropionic acid, we performed a regio- and stereoselective N 2-functionalization of thiosemicarbazones of aromatic and heteroaromatic aldehydes by alkaline hydration of the corresponding (E)-N 2-cyanoethyl derivatives (propanenitriles) prepared by a regioand stereoselective cyanoethylation with acrylonitrile. The hydration proceeds with the retention of the Econfiguration of the initial propanenitriles.
Similar content being viewed by others
References
Youssef, A.S.A., Phosph. Sulfur, Silicon., 2002, vol. 177, p. 173.
Gomaa, M.A.-M., Hassan, A.A., and Shehatta, H.S., Heteroatom Chem., 2006, vol. 17, p. 261.
Alahari, A., Trivelli, X., Guérardel, Y., Dover, L.G., Besra, G.S., Sacchettini, J.C., Reynolds, R.C., Coxon, G.D., and Kremer, L., PLoS ONE, 2007, vol. 2, p. 1343. doi 10.1371/journal.pone.0001343
Kizilcikli, Ý., Kurt, Y.D., Akkurt, B., Genel, A.Y., Birteksöz, S., Ötük, G., and Ülküseven, B., Folia Microbiol., 2007, vol. 52, p. 15.
Darehkordi, A., Saidi, K., and Islami, M.R., Arkivoc, 2007, vol. i, p. 180.
Ðiloviæ, I., Rubèiæ, M., Vrdoljak, V., Paveliæ, S.K., Kralj, M., Piantanidab, I., and Cindriæ, M., Bioorg. Med. Chem., 2008, vol. 16, p. 5189.
De Aquino, T.M., Liesen, A.P., da Silva, R.E.A., Lima, V.T., Carvalho, C.S., de Faria, A.R., de Araujo, J.M., de Lima, J.G., Alves, A.J., de Melo, E.J.T., and Goes, A.J.S., Bioorg. Med. Chem., 2008, vol. 16, p. 446. doi 10.1016/j.bmc.2007.09.025
Matesanz, A.I. and Souza, P., Med. Chem., 2009, vol. 9, p. 1389.
Gazieva, G.A. and Kravchenko, A.N., Russ. Chem. Rev., 2012, vol. 81, p. 494. doi 10.1070/ RC2012v081n06ABEH004235
Ahmadi, S.A. and Ghazanfari, D., Iran. J. Cat., 2013, vol. 3, p. 177.
Singhal, S., Arora, S., Agarwal, S., Sharma, R., and Singhal, N., World J. Pharm. Pharm. Sci., 2013, vol. 2, p. 4661.
Macegoniuk, K., Folia Biol. Oecol., 2013, vol. 9, p. 9. doi 10.2478/fobio-2013-0004
Beraldo, H. and Gambino, D., Mini Rev. Med. Chem., 2004, vol. 4, p. 31.
Tsimberidou, A.-M., Alvarado, Y., and Giles, F.J., Expert Rev. Anticancer Ther., 2002, vol. 2, p. 437. doi 10.1586/14737140.2.4.437
Heffeter, P., Pirker, C., Kowol, C.R., Herrman, G., Dornetshuber, R., Miklos, W., Jungwirth, U., Koellensperger, G., Keppler, B.K., and Berger, W., Leuk. Res., 2003, vol. 27, p. 1077.
Gopalakrishnan, М., Sureshkumar, Р., Thanusu, J., and Kanagarajan, V., Pharm. Chem. J., 2008, vol. 42, p. 271.
Rastogi, S. and Rastogi, H., Ind. J. Chem., 2010, vol. 49B, p.547.
Barcelos, R.P., de Lima Portella, R., da Rosa, E.J.F., de Souza Fonseca, A., Bresolin, L., and Carratu, V., Life Sci., 2011, vol. 89, p. 20.
Aslam, M.A.S., Mahmood, S., Shahid, M., Saeed, A., and Iqbal, J., Eur. J. Med. Chem. 2011, 46, 5473. doi 10.1016/j.ejmech.2011.09.009
Mashkovskii, M.D., Lekarstvennye sredstva (Drugs), Moscow: Novaya Volna, 2003, vol.3.
Kleemann, A., Engel, J., Kutscher, B., and Reichert, D., Pharmaceutical Substances: Syntheses, Patents, Applications, Stuttgart, New York: Thieme, 2001.
Elokhina, V.N., Aleksandrova, A.E., Nakhmanovich, A.S., Shchegoleva, R.A., Karnaukhova, R.V., Vinogradova, T.I., and Kalikhman, I.D., RF Patent no. 1621449, 1989; Byull. Izobret., 1996, no.25.
Nakhmanovich, A.S., Elokhina, V.N., Dolgushin, G.V., Gushchin, A.S., Polyakov, R.A., Volkova, K.A., and Puniya, V.S., RF Patent no. 2265014, 2004, Byull. Izobret., 2005, no.33.
Gushchin, A.S., Vinogradova, T.I., Yablonskii, P.K., Batyunin, G.A., Zabolotnykh, N.V., Vasil’eva, S.N., and Malygin, A.V., RF Patent no. 2423977, 2010; Byull. Izobret., 2011, no.20.
Smolentsev, A.I., Lavrenova, L.G., Elokhina, V.N., Nakhmanovich, A.S., and Larina, L.I., J. Struct. Chem., 2009, vol. 50, p. 500.
Trofimov, B.A., Amosova, S.V., Elokhina, V.N., Yaroshenko, T.I., and Potapov, V.A., RF Patent no. 2476426, 2011; Byull. Izobret., 2013, no.6.
Amit, S., Int. J. Med. Sci. Clin. Inven., 2014, vol. 1, p. 15.
Allegretti, M., Bertini, R., Colotta, F., Caselli, G., Cesta, M.C., Sabbatini, V., and Bizzarri, C., Canad. Patent no. 2420585, 2009; Chem. Abstr., 2001, vol. 135, p. 331442.
Luithle, J., Bob, F.-G., Erb, C., Schnizler, K., Flessner, T., Kampen, M.V., and Methfessel, C., Canad. Patent no. 2479097, 2002; Chem. Abstr., 2003, vol. 139, p. 277049.
Eremeev, A., Kalvinsh, I.Y., Semenikhina, V.G., Liepinsh, E.E., Latvietis, Y.Y., Anderson, P.P., Astapenok, E.B., Spruzh, Y.Y., Trapentsiers, P.T., Podoprigora, G.I., and Giller, S.A., US Patent no. 4481218, 1984; Chem. Abstr., 1981, vol. 94, p. 30198d.
Kalvinsh, I. and Birmans, A., WO App. no. 2005012233, 2005; Chem. Abstr., 2005, vol. 142, p. 218963.
Görgens, C., Guddat, S., Dib, J., Geyer, H., Schänzera, W., and Thevisa, M., Drug Test. Analysis, 2015, vol. 7, p. 973. doi 10.1002/dta.1788
Dambrova, M., Makrecka-Kuka, M., Vilskersts, R., Makarova, E., Kuka, J., and Liepinsh, E., Pharm. Res., 2016, vol. 113, p. 771.
Mal'kina, A.G., Nosyreva, V.V., Albanov, A.I., Afonin, A.V., Vashchenko, A.V., Amosova, S.V., and Trofimov, B.A., Synth. Commun., 2017, vol. 47, p. 159. doi 10.1080/00397911.2016.1257723
Tenório, R.P., Carvalho, C.S., Pessanha, C.S., de Lima, J.G., de Faria, A.R., Alves, A.J., de Melo, E.J.T., and Góes, A.J.S., Bioorg. Med. Chem. Lett., 2005, vol. 15, p. 2575.
Ali, T.E.-S. and Abdel-Monem, W.R., Phosph. Sulfur, Silicon Relat. Elem., 2008, vol. 183, p. 2161.
Afonin, A.V., Ushakov, I.A., Pavlov, D.V., Ivanov, A.V., and Mikhaleva, A.I., Magn. Res. Chem., 2010, vol. 48, p. 685.
Afonin, A.V., Pavlov, D.V., Ushakov, I.A., and Keiko, N.A., Magn. Res. Chem., 2012, vol. 50, p. 502.
Jatav, V., Mishra, P., Kashaw, S., and Stables, J.P., Eur. J. Med. Chem., 2008, vol. 43, p. 135.
Abbasi, A., Gernmayen, S., Taheri, A.N., Shahroosvand, H., and Shabani, M., Eur. J. Chem., 2010, vol. 7, p. 294.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.G. Mal’kina, V.V. Nosyreva, A.V. Afonin, A.I. Albanov, Q.A. Apartsin, E.G. Grigor’ev, B.A. Trofimov, 2017, published in Zhurnal Organicheskoi Khimii, 2017, Vol. 53, No. 8, pp. 1211–1216.
Rights and permissions
About this article
Cite this article
Mal’kina, A.G., Nosyreva, V.V., Afonin, A.V. et al. Regio- and stereoselective N 2-functionalization with propanamide fragment of aromatic and heteroaromatic aldehydes thiosemicarbazones. Russ J Org Chem 53, 1226–1232 (2017). https://doi.org/10.1134/S1070428017080115
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428017080115