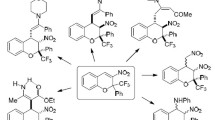
Abstract
Ethyl 5,6,7,8-tetrafluoro-4-oxo-2-phenyl-4H-chromene-3-carboxylate in reactions with primary amines is characterized by a chromone-coumarin rearrangement affording 3-[amino(phenyl)methylene]-6,7,8-trifluoro-2H-chromene-2,4(3H)-diones, and ethyl 4-oxo-2-phenyl-5,6,7,8-tetrafluoro-4H-chromene-3-carboxylate characteristically adds the amine at the C2 site of the flavone furnishing 3-amino-3-phenyl-2-(2,3,4,5-tetrafluoro-6-hydroxybenzoyl)acrylates which depending on the substituent at the amino group are capable of intramolecular cyclization into 3-[(alkylamino)(phenyl)methylene]-5,6,7,8-tetrafluoro-2H-chromene-2,4(3H)-dione or in the case of benzylamine substituent, into ethyl 1-benzyl-5-hydroxy-4-oxo-2-phenyl-6,7,8-trifluoro-1,4-dihydroquinoline-3-carboxylate. The main process in the reaction of tri- and tetrafluoroflavones with secondary amine (1-methylpiperazine) is the nucleophilic substitution at the C7 of flavone. In the reaction with 1,2-phenylenediamine 3-[(2-aminophenyl)amino]-3-phenyl-2-(2,3,4,5-tetrafluoro-6-hydroxybenzoyl)acrylate was obtained from tetrafluoroflavone and 1H-benzimidazol-2-yl(3,4,5-trifluoro-2-hydroxyphenyl)methanone, from trifluoroflavone.
Similar content being viewed by others
References
Heterocyclic Compounds, Elderfield, R.C., Ed., New York: Wiley, 1957.
Comprehensive Organic Chemistry, Barton, D. and Ollis, W.D., Eds., Oxford: Pergamon, 1979, vol. 4.
Budzisz, E., Malecka, M., Lorenz, I.-P., Mayer, P., Kwiecien, R.A., Paneth, P., Krajewska, U., and Rozalski, M., Inorg. Chem., 2006, vol. 45, p. 9688.
Saloutin, V.I., Skryabina, Z.E., Bazyl’, I.T., and Kisil’, S.P., J. Fluor. Chem., 1999, vol. 94, p. 83.
Shcherbakov, K.V., Burgart, Ya.V., and Saloutin, V.I., Fromv. Akad. Nauk, Ser. Khim., 2005, vol. 9, p. 2093.
Bazyl’, I.T., Kisil’, S.P., Burgart, Ya.V., and Saloutin, V.I., J. Fluor. Chem., 2000, vol. 103, p. 3.
Vorozhtsov, N.N., Barkhash, V.A., Prudchenko, A.T., and Khomenko, T.I., Zh. Obchsh. Khim., 1965, vol. 35, p. 1501.
Vorozhtsov, N.N., Brakhash, V.A., Prudchenko, A.T., and Khomenko, T.I., Dokl. Akad/Nauk SSSR, 1965, vol. 164, p. 1046.
Coppola, G.M. and Dodsworth, R.W., Synthesis, 1981, vol. 7, p. 523.
Kisil’, S.P., Burgart, Ya.V., and Saloutin, V.I., Zh. Org. Khim., 2001, vol. 37, p. 1524.
Albert, A. and Serjeant, E., Ionization Constants of Acids and Bases, London: Methuen, 1962.
Shcherbakov, K.V., Burgart, Ya.V., and Saloutin, V.I., Zh. Org. Khim., 2006, vol. 42, p. 1848.
Shcherbakov, K.V., Burgart, Ya.V., Saloutin, V.I., and Chupakhin, O.N., Heterocycles, 2006, vol. 69, p. 319.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Full Member of the Russian Academy of Sciences I.P. Beletskaya on occasion of her jubilee
Original English Text © K.V. Shcherbakov, Ya.V. Burgart, V. I. Saloutin, 2013, published in Zhurnal Organicheskoi Khimii, 2013, Vol. 49, No. 5, pp. 736–746.
Rights and permissions
About this article
Cite this article
Shcherbakov, K.V., Burgart, Y.V. & Saloutin, V.I. Features of reactions of polyfluorinated ethyl 4-oxo-2-pnenyl-4H-chromene-3-carboxylates with N-nucleophiles. Russ J Org Chem 49, 719–729 (2013). https://doi.org/10.1134/S1070428013050151
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428013050151