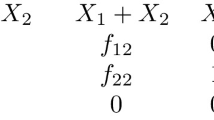Abstract
It is shown that for chemical reactions occurring in an open isothermal gradientless reactor, the stationary state for any stoichiometric total concentration of compounds is unique and stable, even if there are several stationary states for each of the compounds. Relationships are established that allow deriving the exact values of linear and nonlinear relaxation times of reactions, which determine, respectively, the rate and time of reaching a given neighborhood of a stationary state from the total stationary concentration of compounds for any moment of time relying on the stoichiometry of the staged reaction scheme. Examples of calculating the exact values of relaxation times for a number of reactions proceeding according to the kinetic laws of mass action and Marcelin–de Donder equations are considered.



Similar content being viewed by others
REFERENCES
Yablonskii, G.S., Bykov, V.I., and Gorban’, A.N., Kineticheskie modeli kataliticheskikh reaktsii (Kinetic Models of Catalytic Reactions), Novosibirsk: Nauka, 1983.
Bykov, V.I. and Tsybenova, S.B., Nelineinye modeli khimicheskoi kinetiki (Nonlinear Models of Chemical Kinetics), Moscow: URSS, 2011.
Butenin, N.V., Neimark, Yu.I., and Fufaev, N.A., Vvedenie v teoriyu nelineinykh kolebanii (Introduction to the Theory of Nonlinear Oscillations), Moscow: Nauka, 1987.
Andronov, A.A., Vitt, A.A., and Khaikin, S.E., Teoriya kolebanii (Oscillation Theory), Moscow: GI FML, 1959.
Met’yuz, D.G. and Fink, K.D., Chislennye metody. Ispol’zovanie MATLAB (Numerical Methods. Using MATLAB), Moscow: ID Vil’yams, 2001.
Gorban’, A.N., Bykov, V.I., Yablonskii, G.S., Ocherki o khimicheskoi relaksatsii (Essays on Chemical Relaxation), Novosibirsk: Nauka, 1986.
Temkin, M.I., Kinetika Kataliz, 1976, vol. 17, no. 5, pp. 1095–1100.
Fedotov V.Kh., Kolʹtsov, N.I., Gaidai, N.A., Agafonov, Yu.A., Botavina, M.A., Lapidus, A.L., Russ. J. Appl. Chem., 2016, vol. 89, no. 5, pp. 719–726. https://doi.org/10.1134/S1070427216050062
Koltsov, N.I., Russ. J. Appl. Chem., 2021, vol. 94, no. 4, pp. 528–532. https://doi.org/10.1134/S1070427221040142
Fedotov V.Kh. and Koltsov, N.I., Russ. J. Phys. Chem. B, 2014, vol. 8, no. 3, pp. 309–316.
Fedotov, V.Kh. and Kol’tsov, N.I., Izv. vuzov. Khimiya Khim. Tekhnologiya, 2014, vol. 57, no. 2, pp. 63–67.
Korzukhin, M.D., Zh. Fiz. Khim., 1972, vol. 46, no. 7, pp. 1845–1847.
De Donder, T. and Van Rysselberghe, P., Entropy Production Rate for Avascular Tumor Growth, Moscow: Metallurgiya, 1984.
Acknowledgments
The author would like to thank V.Kh. Fedotov.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 4, pp. 437–443, March, 2022 https://doi.org/10.31857/S004446182204003X
Rights and permissions
About this article
Cite this article
Kol’tsov, N.I. Relaxation Times of Chemical Reactions with Arbitrary Kinetics. Russ J Appl Chem 95, 499–505 (2022). https://doi.org/10.1134/S1070427222040048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427222040048