Abstract
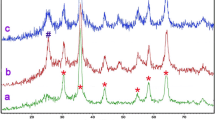
Magnetically recoverable cobalt doping Fe3O4/TiO2 magnetic nanocomposites with an acceptable core–shell structure were prepared via a sol-gel process at low calcination temperature. The crystalline size and structure, morphology, and magnetic properties of resulting particles have been characterized by X-ray diffraction (XRD), fourier transform infrared (FT-IR), FT-Raman, high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), and vibrating sample magnetometry (VSM). Metoprolol tartrate (MET) as a pharmaceutical pollutant was used to observe the photocatalytic degradation ability of the magnetically recoverable particles. The process of degradation under UV irradiation at controlled temperature was studied and the remaining concentrations of MET as a contaminant were measured by UV-Vis spectrometer at λ = 229 nm. This ability remained 95.76% after three times of repetitive use at the same conditions. Various parameters such as reaction temperature, pH, and speed of stirring of the aqueous solution had an effect on the rate of degradation. The amount of cobalt dopant and nanocomposites are also effective on the rate of degradation. Coupling of electrical current with photocatalytic process has proven to be effective in the degradation of MET aqueous solution clearly.
Similar content being viewed by others
References
Romero, V., Marco, P., Giménez, J., and Esplugas, S., Int. J. Photoenergy, 2013, vol. 2013, pp. 1–10.
Farzadkia, M., Bazrafshan, E., Esrafili, A., Yang, J.K., and Siboni, M.S., Iran J. Environ. Health Sci. Eng., 2015, vol. 13, pp. 35–42.
Abramovic, B., Kler, S., Šojic, D., Lauševic, M., Radovic, T., and Vione, D., J. Hazard. Mater., 2011, vol. 198, pp. 123–132.
Yang, H., An, T., Li, G., Song, W., Cooper, W.J., Luo, H., and Guo, X., J. Hazard. Mater., 2010, vol. 179, no. 1–3, pp. 834–839.
Sirés, I. and Brillas, E., Environ. Int., 2012, vol. 40, pp. 212–229.
Arriaga, F.M., Esplugas, S., and Giménez, J., Water Research, 2008, vol. 42, no. 3, pp. 585–594.
Díaz, J.D.M., Joya, G., Utrilla, J.R., Ramos, R.L., Polo, M.S., García, M.A.F., and Castillo, N.A.M., J. Colloid Interface Sci., 2010, vol. 345, no. 2, pp. 481–490.
Choina, J., Kosslick, H., Fischer, C., Flechsig, G.U., Frunza, L., and Schulz, A., Appl. Catal. B, 2013, vol. 129, pp. 589–598.
Li, C., Younesi, R., Cai, Y., Zhu, Y., Ma, M., and Zhu, J., Appl. Catal. B, 2014, vol. 156–157, pp. 314–322.
Hu, S., Li, F., Fan, Z., and Chang, C.C., Appl. Surf. Sci., 2011, vol. 258, no. 1, pp. 182–188.
Qourzal, S., Assabbane, A., and Ichou, Ya., J. Photochem. Photobiol. A, 2004, vol. 163, no. 3, pp. 317–321.
Li, B., Wang, X., Yan, M., and Li, L., Mater. Chem. Phys., 2003, vol. 78, no. 1, pp. 184–188.
Akpan, U.G., and Hameed, B.H., J. Hazard. Mater., 2009, vol. 170, no. 2–3, pp. 520–529.
Zhang, D., High Energ. Chem., 2012, vol. 46, no. 3, pp. 206–211.
Bahadur, N., Jain, K., Srivastava, A.K., Gakhar, G.R., Haranath, D., and Dulat, M.S., Mater. Chem. Phys., 2010, vol. 124, no. 1, pp. 600–608.
Kontos, A.I., Likodimos, V., Stergiopoulos, T., Tsoukleris, D.S., and Falaras, P., Chem. Mater., 2009, vol. 21, no. 4, pp. 662–672.
Khanna, P.K., Singh, N., and Charan, S., Mater. Lett., 2007, vol. 61, no. 25, pp. 4725–4730.
Wang, C., Böttcher, C., Bahnemann, D.W., and Dohrmann, J.K., J. Mater. Chem., 2003, vol. 13, no. 9, pp. 2322–2329.
Karthik, K., Pandian, S.K., and Jaya, N.V., Appl. Surf. Sci., 2010, vol. 256, no. 22, pp. 6829–6833.
Hamadanian, M., Vanani, A.R., and Majedi, A., J. Iran Chem. Soc., 2010, vol. 7, pp. S52–S58.
Minh, N.V., Hien, N.T.M., Vien, V., Kim, S.J., Noh, W.S., Yang, I., Dung, D.T., Khang, N.C., and Khoi, N.T., J. Korean Phys. Soc., 2008, vol. 52, no. 5, pp. 1629–1632.
Santara, B., Pal, B., and Giri, P.K., J Appl. Phys., doi: http://dx.doi.org/10.1063/1.3665883
Mugundan, S., Rajamannan, B., Viruthagiri, G., Shanmugam, N., Gobi, R., and Praveen, P., Appl. Nanosci., 2015, vol. 5, no. 4, pp. 449–456.
Zhu, H., Yang, B., Xu, J., Fu, Z., Wen, M., Guo, T., Fu, S., Zuo, J., and Zhang, S., Appl. Catal. B, 2009, vol. 90, no. 3–4, pp. 463–469.
Yang, L., Luo, S., Li, Y., Xiao, Y., Kang, Q., and Cai, Q., Environ. Sci. Technol., 2010, vol. 44, no. 19, pp. 7641–7646.
Xin, T., Ma, M., Zhang, H., Gu, J., Wang, S., Liu, M., and Zhang, Q., Appl. Surf. Sci., 2014, vol. 288, pp. 51–59.
Pang, S.C., Kho, S.Y., and Chin, S.F., J. Nanomater., doi:10.1155/2012/427310
Kim, H.S., Kim, D., Kwak, B.S., Han, G.B., Um, M.H., and Kang, M., Chem. Eng. J., 2014, vol. 243, pp. 272–279.
Šcepanovic, M., Abramovic, B., Golubovic, A., Kler, S., Brojcin, M.G., Mitrovic, Z.D., Babic, B., Matovic, B., and Popovic, Z.V., J. Sol-Gel Sci. Technol., 2012, vol. 61, no. 2, pp. 390–402.
Rengaraj, S., Venkataraj, S., Yeon, J.W., Kim, Y., Li, X.Z., and Pang, G.K.H., Appl. Catal. B., 2007, vol. 77, no. 1–2, pp. 157–165.
Niu, H., Wang, Q., Liang, H., Chen, M., Mao, C., Song, J., Zhang, S., Gao, Y., and Chen, C., Materials, 2014, vol. 7, no. 5, pp. 4034–4044.
Wei, J., Leng, C.J., Zhang, X., Li, W., Liu, Z.Y., and Shi, J., J. Phys. Conf. Ser., doi 10.1088/1742-6596/149/1/012083
Behrad, F., Farimani, M.H.R., Shahtahmasebi, N., Roknabadi, M.R., and Karimipour, M., EPJ Plus, 2015, vol. 130, pp. 144–152.
Li, Y., Qiu, W., Qin, F., Fang, H., Hadjiev, V.G., Litvinov, D., and Bao, J., J. Phys. Chem. C., 2016, vol. 120, no. 8, pp. 4511–4516.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Teimouri, M., Waqif-Husain, S., Saber-Tehrani, M. et al. Photocatalytic and photoelectrocatalytic degradation of metoprolol tartrate in aqueous media by recyclable Co doping Fe3O4/TiO2 magnetic core–shell nanocomposites. Russ J Appl Chem 90, 1309–1314 (2017). https://doi.org/10.1134/S1070427217080195
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427217080195