Abstract
Metronidazole (MNZ) is a brand of nitroimidazole antibiotic, which is generally used in clinical applications and extensively used for the treatment of infectious diseases caused by anaerobic bacteria and protozoans. The aim of this investigation was to degrade MNZ with illuminated TiO2 nanoparticles at different catalyst dosage, contact time, pH, initial MNZ concentration and lamp intensity. Maximum removal of MNZ was observed at near neutral pH. Removal efficiency was decreased by increasing dosage and initial MNZ concentration. The reaction rate constant (k obs ) was decreased from 0.0513 to 0.0072 min−1 and the value of electrical energy per order (EEo) was increased from 93.57 to 666.67 (kWh/m3) with increasing initial MNZ concentration from 40 to 120 mg/L, respectively. The biodegradability estimated from the BOD5/COD ratio was increased from 0 to 0.098. The photocatalyst demonstrated proper photocatalytic activity even after five successive cycles. Finally, UV/TiO2 is identified as a promising technique for the removal of antibiotic with high efficiency in a relatively short reaction time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Recently, several different types of emerging contaminants in water systems are known as new environmental hazards those need to be treated with suitable methods [1]. As various pharmaceutical compounds have been used since the 1950s due to rapid population growth and development of medical science, several pharmaceutical compounds have been found in surface water, ground water and effluents from wastewater treatment plants. Metronidazole (2-methyl-5-nitroimidazole-1-ethanol) has been widely used to treat infections caused by anaerobic bacteria, bacteroides and protozoa [2-4]. Residual concentrations of metronidazole (MNZ) in surface waters and wastewater are 1 ~ 10 ng/L [5,6]. As MNZ is non-biodegradable and highly soluble in water, it can be accumulated in the aquatic environment [7,8]. Elimination of MNZ from water system is an important issue considering its toxicity, potential mutagenicity and carcinogenity [7,8]. In order to remove MNZ, many techniques such as adsorption [9,10], reduction with nanoscale zero-valent iron particles [11], biological methods [12,13], ozonation technology [14], photolysis [15], Fenton and photo-Fenton processes [16], heterogeneous photocatalysis [15,17,18] and electro-Fenton process with a Ce/SnO2–Sb coated titanium anode [1] have been applied.
Adsorption is widely used method for the treatment of wastewater containing toxic organic compounds. However, it just transfer contaminants from water to a solid phase without any degradation [9,10,19,20]. Biological method is also known as one of the suggested techniques. However this method generally requires long periods for treatment [12,21]. Oxidation is a promising process but sometimes it is regarded as a limited process due to the formation of intermediates with higher toxicity than the parent compound [5,8,22]. Therefore near complete mineralization of MNZ is the most relevant option. For this purpose, advanced oxidation process (AOP) is regarded as a promising option to treat wastewater containing MNZ due to a complete mineralization of parent material as well as lack of selectivity [7,23]. Generally AOPs involve generation of hydroxyl radicals through UV/photocatalyst, UV/H2O2 and UV/O3 processes [24-26]. Among these methods, photocatalytic reaction using TiO2/UV can treat non-biodegradable organic compounds to biodegradable species [23,24,27]. Considering characteristics of the AOP, it can be used as pre- or post-treatment process in wastewater treatment because of its installation easiness in conventional wastewater treatment facilities [23,24,27].
Therefore, in the present work, P-25 TiO2 was selected as a catalyst in the photocatalytic removal of MNZ. Effects of several operational parameters including pH, TiO2 dosage and MNZ concentration on photocatalytic degradation of MNZ were investigated. Kinetic parameters for the photocatalytic degradation were obtained by application of the Langmuir–Hinshelwood (L–H) model. Finally, electrical energy per order (EEo) was obtained to evaluate cost-efficiency of the processes used in this research.
Material and methods
Chemicals
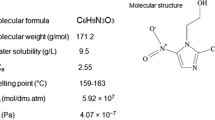
Analytical grade of MNZ (C6H9N3O3; 99% chemical reagent) was purchased from Merck (Darmesdat, Germany) and its physical and chemical characteristics are summarized in Table 1. Potassium dihydrogen phosphate (KH2PO4) and acetonitrile (99.7%, HPLC grade) were purchased from Merck. P-25 TiO2 (80/20 mixture of anatase and rutile) was obtained from Degussa Corp. It has approximately spherical shape and has greater than 99.5% purity. The specific surface area of the TiO2 particles was 50 ± 15 m2/g according to Evonik-Industrial Co. The average size of the TiO2 particles was 21 nm. The antibiotic aqueous solution was prepared by dissolving 1 g of MNZ in 1 L distilled water. The antibiotic aqueous solution was prepared weekly and stored at 4°C. Initial COD and BOD5/COD ratio of 1000 mg/L MNZ was 126 mg/L and approximately 0, respectively.
Figure 1a and b shows X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR) image of TiO2, respectively. The main peaks at 2θ values of 25.367, 37.909, 38.667, 48.158, 54.051, 55.204, 62.817 and 68.976 were correspond to the (101), (004), (112), (200), (105), (211), (204) and (116) planes of P-25 TiO2 (JCPDS card no. 36–1451). FT-IR analysis of TiO2 was performed in the range of 400–4000 1/cm (Figure 1b). The absorption bands at 438 1/cm and 620 1/cm was attributed to the Eg and A2g mode, respectively. The pHZPC of TiO2 nanocatalyst was determined adopting the previously reported method.
Experimental set-up
The experimental reactor used for the photocatalytic degradation of MNZ is shown in Figure 2. The total volume of the reactor was 2 L with working volume of 1 L. The solution in the reactor was constantly stirred via a magnetic stirrer (170 rpm). A 125 W medium-pressure UVC lamp emitting maximum wavelength at 247.3 nm and a low-pressure UV lamp with irradiation intensity 8 W were applied as light sources. The light intensity of the UVC lamp was equal to 1020 μw/cm2 measured by a Spectroline model DRC-100X digital radiometer combined with a DIX-365 radiation sensor (ShokofanTosee, Company, Iran).
Experimental procedure and analysis
In batch experiments, a selected dosage of TiO2 (0.5-3 g/L) was added in 1000 mL of MNZ solution with a certain concentration (40–120 mg/L) at different solution pH ranging from 3 to 11. Initial pH of the solution was adjusted by adding NaOH and HCl (0.1 mol/L) and measured by pH meter (Metron, Switzerland). All runs were performed under ambient conditions for 3 h. During the experiments, the solution in the photoreactor was constantly stirred and kept at constant temperature (25 ± 1°C). The MNZ solutions loaded with TiO2 were equilibrated in the dark for 30 min. After the equilibration period, the UV-lamp was switched on and 10 mL of the solution was taken at distinct time intervals. The aqueous samples were centrifuged (Sigma-301, Germany) at 4000 revolution per minute (rpm) for 10 min to eliminate TiO2 and then measured concentration of residual MNZ. The concentration of residual MNZ was determined by high performance liquid chromatography (HPLC, Waters, USA) equipped with a UV detector at 348 nm. A Diamonsil (R) C18 column (5 μm, 250 mm long × 4.6 mm ID) was used. The data were recorded by a chemistation software. The mobile phase was composed of a mixture of acetonitrile and distilled water (30/70, v/v). The flow speed was set at 1.0 mL/min and 20 μL injections were used [4]. COD was determined by COD reactor model AR851 (HACH, USA) and biodegradability was measured by five-day biochemical oxygen demand (BOD5) according to the Standard Methods [28]. A typical HPLC chromatogram of MNZ is shown in Figure 3.
The removal efficiency (%) is calculated by Eq. (1).
where C0 and C are the concentrations of MNZ at initial and at time t (mg/L), respectively.
All experiments were repeated three times and the average values with error percents were reported.
Results and discussion
Effect of TiO2 dosage
Effect of TiO2 dosage on the photocatalytic degradation of MNZ (80 mg/L) was investigated at pH 7. As shown in Figure 4, degradation efficiency of MNZ was increased from 64.28 to 97.61% by increasing irradiation time from 30 to 180 min at 0.5 g/L TiO2. A greater degradation efficiency of MZN was observed over the entire reaction time at low TiO2 dosage. This phenomena can be explained by the increased blockage of the incident UV light with increasing photocatalyst dosage [18,29]. Since the photocatalytic degradation of MNZ was not much increased over the 0.5 g/L of TiO2, further experiments were performed with 0.5 g/L TiO2. Similar results have been reported by other researchers [23,24,27].
Biodegradability of MNZ was evaluated in this work. To measure the biodegradability, BOD5 and COD values were measured before and after UV irradiation and the ratio of BOD5/COD was used as a biodegradability indicator. After 3 h reaction time, removal efficiency of COD was above 97.6% at all catalyst dosages and the ratio of BOD5/COD increased from 0 to 0.098 as the dosage increased from 0.5 to 3 g/L. This result indicates that MNZ can be changed to more biodegradable products.
Effect of pH
Effect of pH on the photocatalytic degradation of MNZ (80 mg/L) was investigated at constant TiO2 dosage (0.5 g/L) by varying the initial pH of solution. Figure 5 shows that the greatest degradation efficiency was obtained at neutral pH over the entire reaction time. MNZ degradation after 180 min in pH 3, 7 and 11 was 98.2, 99.48 and 97.3%, respectively. Also, COD removal after 180 min in pH 3, 7 and 11 was 97.61, 98.02 and 96.82%, respectively. This trend can be explained by the variation of charges on MNZ as well as on the surface of TiO2 at different solution pH. The pHzpc of TiO2 is determined as 6.52 and pKa value of MNZ is 2.55. Therefore, at acidic pH, both TiO2 and MNZ are positively charged, causing negative effect for the adsorption of MNZ on the surface of TiO2. At neutral pH, no repulsive forces between the TiO2 and MNZ might be developed. At basic pH, both TiO2 and MNZ have negative charges, causing negative effect for the adsorption of MNZ on the surface of TiO2. In this study, even though a distinct removal efficiency of MNZ was not observed at different solution pH, the most effective degradation of MNZ was observed at pH 7. Thus further experiments were performed at neutral pH [4,15,18].
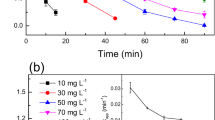
Effect of initial MNZ concentration
Photocatalytic degradation of MNZ by TiO2 was studied by varying the initial MNZ concentration at pH 7 and dosage equal to 0.5 g/L. Figure 6 shows that photocatalytic degradation efficiency decreased as the initial MNZ concentration increased. The presumed reason is that more surface of the TiO2 surface may be occupied by MNZ as the initial MNZ concentration increased. In addition, more degradation intermediates can be accumulated on the TiO2 surface, causing a negative effect in the utilization of hydroxyl radicals or positive holes in the valence band of the TiO2 surface. Moreover, once the concentration of the MNZ increases, more absorption of UV light by MNZ molecules, known as inner filtration effect, can occur. This effect causes decrease of photons reaching to the TiO2 surface [4]. Similar results have been reported by other researchers [15,18,23].
Kinetic study and electrical energy determination
In order to obtain kinetic information, experimental result in Figure 6 was fitted with a pseudo-first-order equation as expressed in Eq. (2).
To calculate the rate constant from the plot ln[C0/C] versus t, only initial data points were considered. Figure 7 shows the plot of ln[C0/C] versus t for the degradation of MNZ. The first-order rate constants of photocatalytic process (kobs (1/min)) at different initial concentrations of MNZ are summarized in Table 2. The relationship between the initial photocatalytic degradation rate (r) and the initial concentration of organic substrate for a heterogeneous photocatalytic process can be described by Langmuir–Hinshelwood (L-H) model (Eqs. 3 and 4) [30,31]:
where [MNZ]0 is the initial concentration of the antibiotic in mg/L, kc (mg/L/min) is the kinetic rate constant of surface reaction and KMNZ (L/mg) is the Langmuir adsorption constant. The values of KMNZ and kc were obtained as 0.0285 L/mg and 0.67 mg/L/min, respectively. This L–H kinetic model has been used by several authors to analyze heterogeneous photocatalytic reactions [30,31].
For the case of photocatalytic reaction, electrical energy is very important factor for the real application and evaluation for the electrical energy should be provided. Thus, in this work, electrical energy was evaluated by calculating electrical energy per order (EEO). It is defined as the number of kWh of electrical energy required to reduce concentration of a pollutant by 1 order of magnitude (90%) in 1 m3 of contaminated water. The EEO (kWh/m3) can be calculated from the following equation:
where P is the rated power (kW) of the AOP system, t is the irradiation time (min), k obs is the pseudo-first order rate constant (1/min), V is the volume (L) of the wastewater in the reactor, Ci and Cf is the initial and final MNZ concentrations, respectively. The EEO value for UV-alone and UV/TiO2 processes are reported in Table 3. EEO value for UV/TiO2 process was lower than UV-alone process.
Comparison of different MNZ removal processes and reusability test
MNZ removal by TiO2-alone, UV 8 W-alone, UV 125 W-alone, UV 8 W/TiO2 and UV 125 W/TiO2 processes are shown in Figure 8. Removal efficiency of MNZ through adsorption process (TiO2-alone) was low. Overall removal efficiency of MNZ by TiO2-alone, UV 8 W-alone, UV 125 W-alone and UV 8 W/TiO2 process was 9.63%, 24.52, 42.32% and 53.53%, respectively. But 99.48% of MNZ was removed with UV 125 W/TiO2. These experiments demonstrate that both UV light and TiO2 are necessary for the effective degradation of MNZ.
Based on the above experiments and analysis, mechanism of the photocatalysis could be proposed as following:
An electron excites from the valence band to the conduction band of TiO2, generating electron–hole pair, with UV light (λ < 390 nm) (Eq. 7) [24,27]:
Then, the generated electron–hole pairs can participate in the reactions with electron acceptors like O2 and donors like H2O or OH− to generate highly reactive radical species particularly hydroxyl radicals (E0 = +3.06 V), which can oxidize organic contaminants and their degradation intermediates unselectively. Furthermore, positive holes can oxidize pollutants directly, too (Eqs. 8–11) [24,27]. Also Homem and Santos [32] reviewed degradation and removal methods of antibiotics from aqueous matrices and suggested removal mechanisms.
The reusability of a photocatalyst is an important factor for real application. Hence, five consecutive photocatalytic experiments were performed by UV/TiO2 process. As can be seen in Figure 9, quite similar photocatalytic activity was maintained over five consecutive runs. Photocatalytic degradation of MNZ with illuminated TiO2 was compared with other reported data. Removal efficiency and reaction rate constant were compared and summarized in Table 4.
Conclusions
From the application of TiO2 for the photocatalytic degradation of MNZ in aqueous solutions, a maximum removal of MNZ was observed at neutral pH. Removal efficiency was decreased by increasing TiO2 dosage and initial MNZ concentration. Electrical energy per order was increased and reaction rate constant was decreased with increasing initial MNZ concentration. Photocatalytic activity was maintained even after five consecutive runs. Finally, UV/TiO2 is identified as a promising technique for the removal of MNZ with high efficiency in a relatively short reaction time.
References
Cheng W, Yang M, Xie Y, Liang B, Fang Z, Tsang EP. Enhancement of mineralization of metronidazole by the electro-Fenton process with a Ce/SnO2–Sb coated titanium anode. Chem Eng J. 2013;220:214–20.
Chatzitakis A, Berberidou C, Paspaltsis I, Kyriakou G, Sklaviadis T, Poulios I. Photocatalytic degradation and drug activity reduction of chloramphenicol. Water Res. 2008;42:386–94.
Dong S, Li Y, Sun J, Yu C, Li Y, Sun J. Facile synthesis of novel ZnO/RGO hybrid nanocomposites with enhanced catalytic performance for visible-light-driven photodegradation of metronidazole. MCP. 2014;145:357–65.
Farzadkia M, Esrafili A, Baghapour MA, Shahamat YD, Okhovat N. Degradation of metronidazole in aqueous solution by nano-ZnO/UV photocatalytic process. Desalin Water Treat. 2013;52:4947–52.
Dantas RF, Rossiter O, Teixeira AKR, Simões AS, da Silva VL. Direct UV photolysis of propranolol and metronidazole in aqueous solution. Chem Eng J. 2010;158:143–7.
Vulliet E, Cren-Olivé C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ Pollut. 2011;159:2929–34.
Zhenhu X, Zaixu C, Jianming L. Comparison of metronidazole degradation by different advanced oxidation processes in low concentration aqueous solutions. Chin J Environ Eng. 2009;3:465–9.
Johnson MB, Mehrvar M. Aqueous metronidazole degradation by UV/H2O2 process in single-and multi-lamp tubular photoreactors: kinetics and reactor design. Ind Eng Chem Res. 2008;47:6525–37.
Méndez-Díaz J, Prados-Joya G, Rivera-Utrilla J, Leyva-Ramos R, Sánchez-Polo M, Ferro-García MA, et al. Kinetic study of the adsorption of nitroimidazole antibiotics on activated carbons in aqueous phase. JCIS. 2010;345:481–90.
Rivera-Utrilla J, Prados-Joya G, Sánchez-Polo M, Ferro-García M, Bautista-Toledo I. Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon. J Hazard Mater. 2009;170:298–305.
Fang Z, Chen J, Qiu X, Qiu X, Cheng W, Zhu L. Effective removal of antibiotic metronidazole from water by nanoscale zero-valent iron particles. Desalination. 2011;268:60–7.
Ingerslev F, Halling-Sørensen B. Biodegradability of metronidazole, olaquindox, and tylosin and formation of tylosin degradation products in aerobic soil–manure slurries. Ecotoxicol Environ Saf. 2001;48:311–20.
Ingerslev F, Toräng L, Loke M-L, Halling-Sørensen B, Nyholm N. Primary biodegradation of veterinary antibiotics in aerobic and anaerobic surface water simulation systems. Chemosphere. 2001;44:865–72.
Sánchez-Polo M, Rivera-Utrilla J, Prados-Joya G, Ferro-García M, Bautista-Toledo I. Removal of pharmaceutical compounds, nitroimidazoles, from waters by using the ozone/carbon system. Water Res. 2008;42:4163–71.
Prados-Joya G, Sánchez-Polo M, Rivera-Utrilla J, Ferro-Garcia M. Photodegradation of the antibiotics nitroimidazoles in aqueous solution by ultraviolet radiation. Water Res. 2011;45:393–403.
Shemer H, Kunukcu YK, Linden KG. Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere. 2006;63:269–76.
Gao J, Liu B, Wang J, Jin X, Jiang R, Liu L, et al. Spectroscopic investigation on assisted sonocatalytic damage of bovine serum albumin (BSA) by metronidazole (MTZ) under ultrasonic irradiation combined with nano-sized ZnO. Spectrochim Acta A Mol Biomol Spectrosc. 2010;77:895–901.
Wang H, Zhang G, Gao Y. Photocatalytic degradation of metronidazole in aqueous solution by niobate K6Nb10.8O30. Wuhan Univ J Nat Sci. 2010;15:345–9.
Ahmed MJ, Theydan SK. Microporous activated carbon from Siris seed pods by microwave-induced KOH activation for metronidazole adsorption. J Anal Appl Pyrolysis. 2013;99:101–9.
Çalışkan E, Göktürk S. Adsorption characteristics of sulfamethoxazole and metronidazole on activated carbon. SS&T. 2010;45:244–55.
Saidi I, Soutrel I, Floner D, Fourcade F, Bellakhal N, Amrane A, et al. Indirect electroreduction as pretreatment to enhance biodegradability of metronidazole. J Hazard Mater. 2014;278:172–9.
Jimmy C. In situ synthesis of Zn2GeO4 hollow spheres and their enhanced photocatalytic activity for the degradation of antibiotic metronidazole. DTr. 2013;42:5092–9.
Palominos RA, Mondaca MA, Giraldo A, Peñuela G, Pérez-Moya M, Mansilla HD. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal Today. 2009;144:100–5.
Elmolla ES, Chaudhuri M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination. 2010;252:46–52.
Giraldo AL, Penuela GA, Torres-Palma RA, Pino NJ, Palominos RA, Mansilla HD. Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspension. Water Res. 2010;44:5158–67.
Farrokhi M, Yang J-K, Lee S-M, Shirzad-Siboni M. Effect of organic matter on cyanide removal by illuminated titanium dioxide or zinc oxide nanoparticles. J Environ Health Sci Eng. 2013;11:23.
Dimitrakopoulou D, Rethemiotaki I, Frontistis Z, Xekoukoulotakis NP, Venieri D, Mantzavinos D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J Environ Manage. 2012;98:168–74.
Federation WE, Association APH. Standard methods for the examination of water and wastewater. Washington, DC, USA: American Public Health Association (APHA); 2005.
Wang J, Wang Z, Jin X, Guo Y, Gao J, Li K, et al. Catalytic damage of bovine serum albumin by metronidazole under ultrasonic irradiation in the presence of nano-sized TiO2 powder. Rus J Physic Chem A. 2012;86:867–74.
Daneshvar N, Salari D, Khataee AR. Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J Photochem Photobiol A Chem. 2003;157:111–6.
Daneshvar N, Salari D, Khataee AR. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol A Chem. 2004;162:317–22.
Homem V, Santos L. Degradation and removal methods of antibiotics from aqueous matrices - A review. J Environ Manage. 2011;92:2304–47.
Acknowledgment
The authors thank the Iran and Zahedan Universities of Medical Sciences, Iran for all of the support provided. Also, authors would like to thank Mr. Bonyani for the HPLC analysis in the laboratory of nutrition department.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
MF carried out the degradation studies of Metronidazole. EB contributed in writing of the manuscript and analyzing of data. AE involved in design of removal experiments, analyzing of data. J-KY discussed XRD and FT-IR data of titanium dioxide and participated in the sequence alignment of the manuscript. MS-S involved in design of removal experiments, analyzing of data, writing of the manuscript and reviewing of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farzadkia, M., Bazrafshan, E., Esrafili, A. et al. Photocatalytic degradation of Metronidazole with illuminated TiO2 nanoparticles. J Environ Health Sci Engineer 13, 35 (2015). https://doi.org/10.1186/s40201-015-0194-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40201-015-0194-y