Abstract
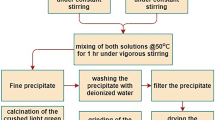
The present work focuses on the synthesis of high surface area NiO nanoparticles through thermal decomposition of [Ni(binol)(bpy)]∙CH3OH complex as a new precursor. [Ni(binol)(bpy)]∙CH3OH (where binol = racemic-1,1′-bi-2-naphtholate and bpy = 2,2′-bipyridine) was synthesized from reaction of NiCl2(bpy) with rac-Na2(binol). The complex was characterized by elemental analysis and spectroscopy techniques of IR, UV-Vis, mass, 1H and 13C NMR. The results revealed that [Ni(binol)(bpy)]∙CH3OH was a paramagnetic tetrahedral complex. The physicochemical properties of the nanoparticles were characterized by various analysis techniques such as X-ray diffraction (XRD), Fourier transform infrared (FTIR), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), and BET specific surface area. The used synthetic rout is facile and economic that makes it suitable for large scale production of pure nickel oxide nanoparticles.
Similar content being viewed by others
References
Loudon, J.C., Phy. Rev. Lett., 2012, vol. 109, pp. 267404–267408.
Cheng, J., Deng, L., Zhang, B., Shi, P., and Meng, G., Rare Met., 2007, vol. 26, p. 110.
Granqvist, C.G., Niklasson, C.G., and Azens, A., Appl. Phys. A, 2007, vol. 89, p. 29.
Lee, C.Y., Chiang, C.M., Wang, Y.H., and Ma, R.H., Sensor Actuator B: Chem., 2007, vol. 122, p. 503.
Ji, Z., Natu, G., and Wu, Y., ACS Appl. Mater. Interfaces, 2013, vol. 5, pp. 8641–8648.
Nitin, C., Sunayana, S., Sharma, M.K., and Chaturvedi, R.K., Int. J. Res. Chem. Environ., 2011, vol. 1, pp. 66–70.
Reddy, M.V., Subba Rao, G.V.B., and Chowdari, V.R., Chem. Rev., 2013, vol. 113, pp. 5364–5457.
Rakshit, S., Gosh, S., Chall, S., Mati, S.S., Moulik, S.P., and Bhattacharya, S.C., RSC Adv., 2013, vol. 3, pp. 19348–19356.
Yu, F., Xu, X., Peng, H., Yu, H., Dai, Y., Liu, W., Ying, J., Sun, Q., and Wang, X., Appl. Catal. A: Gen., 2015, vol. 507, pp. 109–118.
Liu, J., Peng, H., Liu, W., Xu, X., Wang, X., Li, C., Zhou, W., Yuan, P., Chen, X., Zhang, W., and Zhan, H., Chem. Cat. Chem., 2014, vol. 6, pp. 2095–2104.
Ma, Y., Wang, X., You, X., Liu, J., Tian, J., Xu, X., Peng, H., Liu, W., Li, C., Zhou, W., Yuan, P., and Chen, X., Chem. Cat. Chem., 2014, no. 6, pp. 3366–3376.
You, X., Wang, X., Ma, Y., Liu, J., Liu, W., Xu, X., Peng, H., Li, C., Zhou, W., Yuan, P., Chen, X., and Zhan, H., Chem. Cat. Chem., 2014, vol. 6, pp. 3377–3386.
Sharma, J.K., Srivastava, P., Singh, G., Akhtar, M.S., and Ameen, S., Ceram. Int., 2015, vol. 41, pp. 1573–1578.
Wang, J., Wei, C., Pang, H., Gao, F., Yin, J., Guan, L., and Lu, Q., Catal. Commun., 2011, vol. 12, pp. 1031–1036.
Chen, L-J., Li, G-S., Qi, P., and Li, L-P., J. Therm. Anal. Calorim., 2008, vol. 92, pp. 765–769.
Wang, Y., Zhu., J., Yang, X., Lu, L., and Wang, X., Thermochim. Acta, 2005, vol. 437, pp. 106–109.
Guimon, C., Appl. Catal. A, 2003, vol. 251, pp. 199–214.
Carnes, C.L. and Klabunde, K.J., J. Mol. Catal. A: Chem., 2003, vol. 194, p. 227.
Heracleous, E. and Lemonidou, A., J. Catal., 2006, vol. 237, pp. 175–189.
Litvishkov, Y., Talyshinskii, R., Efendiev, M., and Muradova, P., Petrol. Chem., 2012, vol. 52, pp. 186–188.
Derakhshi, M., Jamali, T., Elyasi, M., Bijad, M., Sadeghi, R., Kamali, A., Niazazari, K., Shahmiri, M.R., Bahari, A., and Mokhtari, S., Int. J. Electrochem. Sci., 2013, vol. 8, pp. 8252–8263.
Deng, X.Y. and Chen, Z., Mater. Lett., 2004, vol. 58, p. 276.
Lang, J.W., Kong, L.B., Wu, W.J., Luo, Y.C., and Kang, L., Chem. Commun., 2008, vol. 35, pp. 4213–4215.
Wang, W.Z., Liu, Y.K., Xu, C.K., Zheng, C.G., and Wang, G.G., Chem. Phys. Lett., 2002, vol. 362, p. 119.
Yang, Q., Sha, J., Ma, X.Y., and Yang, D.R., Mater. Lett., 2005, vol. 59, p. 1967.
Zayim, E.O., Turhan, I., Tepehan, F.Z., Ozer, N., Solar Energy Materials & Solar Cells, 2008, vol. 92, p. 164–169.
Alagiri, M., Ponnusamy, S., and Muthamizhchelvan, C., J. Mater. Sci. Mater. Electron., 2012, vol. 23, pp. 728–732.
Ansar, A., Soysal, D., and Schiller, G., Int. J. Energy Res., 2009, vol. 33, pp. 1191–1202.
Salavati-Niasari, M. and Entesari, M., Polyhedron, 2012, vol. 33, pp. 302–309.
Reinert, F., Steiner, P., Hufner, H., Schimtt, J., Fink, J., Knupper, M., Sandal, P., and Bertel, E., Z. Phys., 1995, B97, pp. 83–93.
Heda, R., Rani, A., Devra, V., and Amritphale, S.S., Int. Res. J. Pure Appl. Chem., 2013, vol. 3, pp. 111–117.
Han, D.Y., Yang, H.Y., Shen, C.B., Zhou, X., and Wang, F.W., Powder Technol., 2004, vol. 147, pp. 113–116.
Palanisamy, P. and Raichur, A.M., Mater. Sci. Eng. C, 2009, vol. 29, pp. 199–204.
Wang, Y.D., Ma, C.L., Sun, X.D., and Li, H.D., Inorg. Chem. Commun., 2002, vol. 5, pp. 751–755.
Deki, S., Yanagimito, H., and Hiraoka, S., Chem. Mater., 2003, vol. 15, pp. 4916–4922.
Kalam, A., Al-Sehemi, A.G., Al-Shihri, A.S., Du, G., and Ahmad, T., Mater. Charact., 2012, 68, pp. 77–81.
Wei, Z., Qiao, H., Yang, H., Zhang, C., and Yan, X., J. Alloys Compd., 2009, vol. 479, pp. 855–858.
Lai, T.L., Shub, Y.Y., Huangb, G.L., Lee, C.C., and Wang, C.B., J. Alloys Compd., 2008, vol. 450, pp. 318–322.
Sun, W., Chen, L., Meng, S., Wang, Y., Li, H., Han, Y., and Wei, N., Mater. Sci. Semicond. Process, 2014, vol. 17, pp. 129–133.
Rahman, M.M., Chou, S.L., Zhong, C., Wang, J.Z., Wexler, D., and Liu, H.K., Solid State Ionics, 2010, vol. 180, pp. 1646–1651.
Ramasami, A.K., Reddy, M.V., and Balakrishna, G.R., Mater. Sci. Semicond. Process, 2015, vol. 40, pp. 194–202.
Khansari, A., Enhessari, M., and Salavati-Niasari, M., J. Clust. Sci., 2013, vol. 24, pp. 289–297.
Farhadi, S., Kazem, M., and Siadatnasab, F., Polyhedron, 2011, vol. 30, pp. 606–613.
Farhadi, S. and Roostaei-Zaniyani, Z., Polyhedron, 2011, vol. 30, pp. 971–975.
Farhadi, S. and Roostaei-Zaniyani, Z., Polyhedron, 2011, vol. 30, pp. 1244–1249.
Mehdizadeh, R., Sanati, S., and Saghatforoush, L.A., Synth. React. Inorg. Metal-Org. Nano-Metal Chem., 2013, vol. 43, pp. 466–470.
Ding, K., Wang, Y., Zhang, L., and Wu, Y., Tetrahedron, 1996, vol. 52, p. 1005.
Miessler, G.L. and Tarr, D.A., Inorganic Chemistry, 4th ed., Upper Saddle River, Prentice Hall, 2011.
Srivastava, D.N., Pol, V.G., Palchik, O., Zhang, L., Yu, J.C., and Gedanken, A., Ultrason. Sonochem., 2005, vol. 12, p. 205.
Fazlali, F., Mahjoub, A., and Abazari, R., Solid State Sci., 2015, vol. 48, pp. 263–269.
Wang, C., Shao, C., Wang, L., Zhang, L., Li, X., and Liu, Y., Colloid Interface Sci., 2009, vol. 333, p. 242.
Christy, A.J. and Umadevi, M., Mat. Res. Bull., 2013, vol. 48, pp. 4248–4254.
Wu, Y., He, Y., Wu, T., Weng, W., and Wan, H., Mat. Let, 2007, vol. 61, pp. 2679–2682.
Bai, G., Dai, H., Deng, J., et al., Chem. Eng. J., 2013, vol. 219, pp. 200–208.
Al-Sehemi, A.G., Al-Shihri, A.S., Kalam, A., Du, G., and Ahmad, T., J. Mol. Structure, 2014, vol. 1058, pp. 56–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the author in English.
Rights and permissions
About this article
Cite this article
Salehirad, A. Synthesis of high surface area NiO nanoparticles through thermal decomposition of mixed ligand Ni(II) Complex, [Ni(binol)(bpy)]∙CH3OH. Russ J Appl Chem 89, 63–69 (2016). https://doi.org/10.1134/S10704272160010109
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S10704272160010109