Abstract
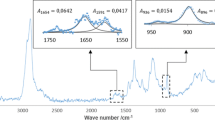
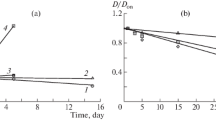
Specific features of formation and the composition and characteristics of dispersed phase particles in mixed dilute solutions of chitosan and magnesium sulfate, containing no surfactants, were studied. The component molar ratio, \(\nu _{SO_4 } :\nu _{NH_2 }\), is the factor exerting the strongest influence on the formation rate and characteristics of chitosan sulfate particles. With an increase in the fraction of sulfate ions in the initial solution, the particle size decreases and their ζ-potential increases. The \(\nu _{SO_4 } :\nu _{NH_2 }\) molar ratio in chitosan sulfate particles only slightly depends on the composition of the initial mixed solution and varies within 0.40–0.44.
Similar content being viewed by others
References
Fernandes, A.L.P., Morais, W.A., and Santos, A.I.B., Colloid Polym. Sci., 2005, vol. 284, no. 1, pp. 1–9.
Loh Jing, W., Schneider, J., Carter, M., et al., J. Pharm. Sci., 2010, vol. 99, no. 10, pp. 4326–4336.
Berthold, A., Cremer, R., and Kreuter, J., J. Control. Release, 1996, vol. 39, pp. 19–25.
Trindade Neto, C.G., Fernandes, A.L.P., Santos, A.I.B., et al., Polym. Int., 2005, vol. 54, no. 4, pp. 659–666.
Il’ina, A.V., Varlamov, V.P., Ermakov, U.A., et al., Dokl. Earth Sci., 2008, vol. 421, no. 2, pp. 190–192.
Il’ina, A.V., Zubareva, A.A., Kurek, D.V., et al., Nanotechnol. Russ., 2012, vol. 7, no. 1, pp. 85–92.
Hejazi, R. and Amiji, M., Int. J. Pharm., 2002, vol. 235, nos. 1–2, pp. 87–94.
Lipatova, I.M., Russ. J. Gen. Chem., 2013, vol. 83, no. 1, pp. 83–91.
Wang, W., Bo, S., Li, S., and Qin, W., Int. J. Biol. Macromol., 1991, vol. 13, pp. 381–387.
Glazunov, V.P. and Gorbach, V.I., Sov. J. Bioorg. Chem., 1999, vol. 25, no. 3, pp. 191–193.
Mironov, A.V., Vikhoreva, G.A., Kil’deeva, N.R., and Uspenskii, S.A., Polym. Sci., Ser. B, 2007, vol. 49, nos. 1–2, pp. 15–17.
Gamzazade, A.I., Sklyar, A.M., Pavlova, S.A., and Rogozhin, S.V., Polym. Sci., 1981, vol. 23, p. 665.
Tavares, I.S., Caroni, A.L.P.F., Dantas Neto, A.A., et al., Colloids Surf. B: Biointerfaces, 2012, vol. 90, pp. 254–258.
Bergstrom, E., Goodall, D.M., and Norton, I.T., Gums and Stabilisers for the Food Industry, Phillips, G.O., Williams P.A., and Wedlock, D.J., Eds., Oxford: IRL, 1990, p. 501.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Mezina, I.M. Lipatova, 2014, published in Zhurnal Prikladnoi Khimii, 2014, Vol. 87, No. 6, pp. 821–827.
Rights and permissions
About this article
Cite this article
Mezina, E.A., Lipatova, I.M. Formation of the dispersed phase in mixed solutions of chitosan and magnesium sulfate. Russ J Appl Chem 87, 830–835 (2014). https://doi.org/10.1134/S1070427214060275
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427214060275