Abstract
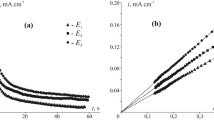
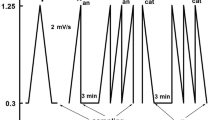
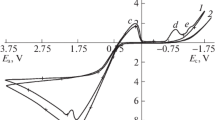
Potentiodynamic polarization and potentiostatic electrolysis techniques were employed to study the anodic oxidation of silver and a number of other metals (copper, iron, nickel, and tin) in sulfite media, both individually and in Ag-M binary electrodes. The conditions in which silver is dissolved with a nearly 100% current efficiency were found.
Similar content being viewed by others
References
Vyacheslavov, P.M., Grilikhes, S.Ya., Burkat, G.K., and Kruglova, E.G., Gal’vanotekhnika blagorodnykh i redkikh metallov (Electroplating of Noble and Rare Metals), Leningrad: Mashinostroenie, 1970.
Smirnov, Yu.E., Vasilenko, K.V., Frolov, V.N., et al., Metallurgiya vtorichnykh dragotsennykh metallov (Metallurgy of Secondary Precious Metals), Novosibirsk: Pasman i Shuvalov. 1996.
Maslenitskii, I.N. and Chugaev, L.V., Metallurgiya blagorodnykh metallov (Metallurgy of Noble Metals), Moscow: Metallurgiya, 1972.
Plaksin, I.N. and Kozhukhova, M.A., Doklady Akad. Nauk SSSR, 1941, vol. 31, pp. 671–674.
Meretukov, M.A. and Orlov, A.M., Metallurgiya blagorodnykh metallov: Zarubezhnyi opyt (Metallurgy of Noble Metals: Foreign Experience), Moscow: Metallurgiya, 1991.
Lesnykh, N.N., Marshakov, I.K., Volkova, L.E., and Tutukina, N.M., Kondens. Sredy Mezhfazn.Granitsy, 2006, vol. 8, no. 2, pp. 125–130.
Marshakov, I.K., Lesnykh, N.N., and Tutukina, N.M., Kondens. Sredy Mezhfazn.Granitsy, 2007, vol. 9, no. 3, pp. 228–233.
Marshakov, I.K., Lesnykh, N.N., and Tutukina, N.M., Kondens. Sredy Mezhfazn.Granitsy, 2007, vol. 9, no. 3, pp. 234–239.
Marshakov, I.K., Lesnykh, N.N., and Tutukina, N.M., Kondens. Sredy Mezhfazn.Granitsy, 2008, vol. 10, no. 2, pp. 132–138.
Lesnykh, N.N., Tutukina, N.M., and Marshakov, I.K., Kondens. Sredy Mezhfazn.Granitsy, 2008, vol. 10, no. 4, pp. 249–255.
Dignam, J., Barrett, M., and Nagy, D., Canad. J. Chem., 1969, vol. 47, pp. 4253–4266.
Zaky, M., Assaf, H., and Abd El Rehim, S., Appl. Surface Sci., 2004, vol. 221, pp. 349–357.
Tomilov, A.P., Prikladnaya elektrokhimiya (Applied Electrochemistry), Moscow: Khimiya, 1984, 3rd ed.
Kakovskii, I.A. and Potashnikov, Yu.M., Kinetika protsessov rastvoreniya (Kinetics of Dissolution Processes), Moscow: Metallurgizdat, 1975.
Potashnikov, Yu.M., Kakovskii, I.A., and Chursanov, Yu.V., Izv. Akad. Nauk SSSR, Metal., 1985, no. 6, pp. 39–45.
Bersirova, O., Tsesiulis, G., and Kublanovskii, V, Rus. J. Appl. Chem., 2009, vol. 82, no. 7, pp. 1222–1225.
Tarasevich, M.R., Chernyshova, L.S., Bogdanovskaya, V.A., and Kudaikulova, G.A., Rus. J. Electrochem., 2001, vol. 37, no. 4, pp. 440–442.
Stability constants of Metal-Ion Complexes: Special Publication no. 17, Compiled by L.G. Sillen, London: The Shem. Soc., Burlington house, W, 1964.
Dobos, D, Electrochemical Data. A Handbook for Electrochemists in Industry and Universities, Budapest: Akademiai Kiado, 1978.
Gladkii, A.V., Zh. Prikl. Khim., 1984, vol. 57, no. 9, pp. 2121–2123.
Khasanov, V.V., Ryzhova, G.L., and Mal’tseva, E.V., Khim. Rast. Syr’ya, 2004, no. 3, pp. 77–85.
Kolthoff, I.M., Belcher, R., Stenger, V.A., and Matsuyama, G., Volumetric analysis, New York: Intersci., 1957, 2nd ed., vol. 3.
Bek, R.Yu., Shuraeva, L.I., Ovchinnikova, S.N., and Vais, A.A., Rus. J. Electrochem., 2007, vol. 43, no. 3, pp. 288–295.
Bek, R.Yu., Shuraeva, L.I., Ovchinnikova, S.N., and Kenzin, V.I, Rus. J. Electrochem., 2007, vol. 43, no. 11, pp. 1260–1267.
Bek, R.Yu. and Shevtsova, O.N., Rus. J. Electrochem., 2011, vol. 47, no. 3, pp. 248–255.
Hiskey, B. and Sanchez, V.M., J. Appl. Electrochem., 1990, vol. 20, pp. 479–487.
Pyatnitskii, I.V. and Sukhan, V.V., Analiticheskaya khimiya serebra (Analytical Chemistry of Silver), Moscow: Nauka, 1975.
Spravochnik po elektrokhimii (Handbook of Electrochemistry), Sukhotin, A.M., Ed., Leningrad: Khimiya, 1981.
Marshakov, I.K., Volkova, L.E., and Tutukina, N.M., Kondens. Sredy Mezhfazn. Granitsy, 2005, vol. 7, no. 4, pp. 417–423.
Marshakov, I.K., Volkova, L.E., and Tutukina, N.M., Kondens. Sredy Mezhfazn.Granitsy, 2006, vol. 8, no. 1, pp. 36–41.
Marshakov, I.K., Lesnykh, N.N., Tutukina, N.M., and Volkova, L.E., Kondens. Sredy Mezhfazn.Granitsy, 2007, vol. 9, no. 2, pp. 138–141.
Aleksandrova, T.P., Ovchinnikova, S.N., Vais, A.A., and Bek, R.Yu., J. Anal. Chem., 1999, vol. 54, no. 7, pp. 646–650.
Author information
Authors and Affiliations
Additional information
Original Russian Text © D.B. Kal’nyi, V.V. Kokovkin, I.V. Mironov, 2012, published in Zhurnal Prikladnoi Khimii, 2012, Vol. 85, No. 1, pp. 60–64.
Rights and permissions
About this article
Cite this article
Kal’nyi, D.B., Kokovkin, V.V. & Mironov, I.V. On anodic dissolution of silver coatings deposited on base metals. Russ J Appl Chem 85, 57–61 (2012). https://doi.org/10.1134/S1070427212010119
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427212010119