Abstract
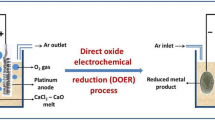
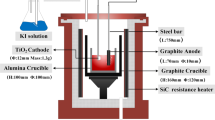
Preparation of titanium by the electrochemical reduction of titanium dioxide in a CaCl2-CaO melt in a diaphragm electrolyzer using a graphite anode was studied.
Similar content being viewed by others
References
Chen, G.Z., Fray, D.J., and Farthing, T.W., Nature, 2000, vol. 407, pp. 361–364.
Chen, G.Z., and Fray, D.J., Progress in Molten Salt Chemistry 1: Prof. N.J. Bjerrum Special Volume, Berg, R.W., and Hjuler, H.A., Eds., Elsevier, 2000, pp. 157–467.
Chen, G.Z., and Fray, D.J., J. Electrochem. Soc., 2002, vol. 149, no. 11, pp. 455–467.
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.A. Lebedev, V.I. Sal’nikov, I.A. Sizikov, D.A. Rymkevich, 2007, published in Zhurnal Prikladnoi Khimii, 2007, Vol. 80, No. 9, pp. 1467–1472.
Rights and permissions
About this article
Cite this article
Lebedev, V.A., Sal’nikov, V.I., Sizikov, I.A. et al. Mechanism and kinetics of processes occurring at TiO2 cathode in CaCl2-CaO melt. Russ J Appl Chem 80, 1503–1508 (2007). https://doi.org/10.1134/S1070427207090121
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S1070427207090121