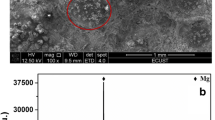
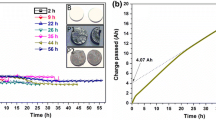
Abstract
Molten CaCl2–CaO system is a suitable electrolyte medium for reducing metal oxides to metal by in-situ electro-generated calcium metal. The graphite anode, which acts as a reactive anode in this melt, leads to several parasitic reactions and decreases the current efficiency. The present study investigates the usage of platinum anode towards the direct oxide electrochemical reduction of ThO2 and NiO by electro-generated calcium in CaCl2–1 wt % CaO melt at 900 °C. Primarily, the anodic behavior of platinum electrode was investigated using electrochemical techniques such as linear sweep voltammetry, potentiodynamic polarization, electrochemical impedance spectroscopy, cyclic voltammetry and potentiostatic electrolysis in CaCl2–CaO melt. Platinum exhibited the most anodic polarization potential, positive corrosion potential, and highest oxidation resistance compared to nickel and gold. It also showed a clearly demarcated potential window for oxygen evolution and much better physico-chemical stability compared to gold electrode. Electro-calciothermic reduction experiments conducted with ThO2 and NiO cathodes using platinum anode demonstrated the feasibility of metallization, and platinum's mass loss rate in these experiments was found to be much less (in the order of ~ 0.016 g cm−2 h−1). The study showed that platinum could be used as an anode in the electrochemical reduction of solid metal oxides in CaCl2–1 wt % CaO melt with minimal mass loss.
Graphical abstract

















Similar content being viewed by others
References
Chen GZ, Fray DJ, Farthing TW (2000) Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 407:361–364. https://doi.org/10.1038/35030069
Ono K, Suzuki RO (2002) A new concept for producing Ti sponge: calciothermic reduction. JOM 54:59–61. https://doi.org/10.1007/BF02701078
Abdelkader AM, Kilby KT, Cox A, Fray DJ (2013) DC voltammetry of electro-deoxidation of solid oxides. Chem Rev 113:2863–2886. https://doi.org/10.1021/cr200305x
Xiao W, Wang D (2014) The electrochemical reduction processes of solid compounds in high temperature molten salts. Chem Soc Rev 43:3215–3228. https://doi.org/10.1039/C3CS60327J
Chen GZ, Fray DJ (2020) Invention and fundamentals of the FFC Cambridge process. Extr Metall Titan Conv Recent Adv Extr Prod Titan Met. https://doi.org/10.1016/B978-0-12-817200-1.00011-9
Suzuki RO, Noguchi H, Haraguchi Y, Natsui S, Kikuchi T (2018) (Invited) Metal production in CaCl2-based melts. ECS Trans 86:45. https://doi.org/10.1149/08614.0045ECST
Suzuki RO, Natsui S, Kikuchi T (2020) OS process: calciothermic reduction of TiO2 via CaO electrolysis in molten CaCl2. Extr Metall Titan Conv Recent Adv Extr Prod Titan Met. https://doi.org/10.1016/B978-0-12-817200-1.00012-0
Osaki S, Sakai H, Suzuki RO (2010) Direct production of Ti–29Nb–13Ta–4.6Zr biomedical alloy from oxide mixture in molten CaCl2. J Electrochem Soc 157:117. https://doi.org/10.1149/1.3435302/XML
Enmei R, Kikuchi T, Suzuki RO (2013) Production of Nb–Ti–Ni alloy in molten CaCl2. Electrochim Acta 100:257–260. https://doi.org/10.1016/J.ELECTACTA.2012.11.044
Suzuki RO, Teranuma K, Ono K (2003) Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall Mater Trans B 34:287–295. https://doi.org/10.1007/S11663-003-0074-1/METRICS
Suzuki RO, Fukui S (2004) Reduction of TiO2 in molten CaCl2 by Ca deposited during CaO electrolysis. Mater Trans 45:1665–1671
Chen GZ (2020) Interactions of molten salts with cathode products in the FFC Cambridge process. Int J Miner Metall Mater 27:1572–1587. https://doi.org/10.1007/s12613-020-2202-1
Sri Maha Vishnu D, Sure J, Mohandas KS (2015) Corrosion of high density graphite anodes during direct electrochemical de-oxidation of solid oxides in molten CaCl2 medium. Carbon 93:782–792. https://doi.org/10.1016/j.carbon.2015.05.093
Ueda I, Baba M, Kikuchi T, Suzuki RO (2013) Formation of niobium powder by electrolysis in molten salt. Electrochim Acta 100:269–274. https://doi.org/10.1016/J.ELECTACTA.2013.01.054
Jones AH, Watson R, Paget T, Campbell-Kelly R, Caldwell T, Fray DJ (2015) Electrochemical reduction of plutonium oxide in molten CaCl2-CaO. J Nucl Fuel Cycle Waste Technol 13:1–5. https://doi.org/10.7733/jnfcwt.2015.13.s.1
Mukherjee A, Kumaresan R, Joseph K (2021) Studies on direct electrochemical de-oxidation of solid ThO2 in calcium chloride based melts. J Electrochem Soc 168:72502. https://doi.org/10.1149/1945-7111/ac0ec8
McGregor K, Frazer E, Urban A, Pownceby M, Deutscher R (2006) Development of inert anode materials for electrowinning in calcium chloride melts. ECS Trans 2:369–380. https://doi.org/10.1149/1.2196026
Ge J, Zou X, Almassi S, Ji L, Chaplin BP, Bard AJ (2019) Electrochemical production of Si without generation of CO2 based on the use of a dimensionally stable anode in molten CaCl2. Angew Chem Int Ed 58:16223–16228. https://doi.org/10.1002/anie.201905991
Barnett R, Kilby KT, Fray DJ (2009) Reduction of tantalum pentoxide using graphite and tin-oxide-based anodes via the FFC-Cambridge process. Metall Mater Trans B 40:150–157. https://doi.org/10.1007/s11663-008-9219-6
Kilby KT, Jiao S, Fray DJ (2010) Current efficiency studies for graphite and SnO2-based anodes for the electro-deoxidation of metal oxides. Electrochim Acta 55:7126–7133. https://doi.org/10.1016/j.electacta.2010.06.049
Jiao S, Fray DJ (2009) Development of an inert anode for electrowinning in calcium chloride-calcium oxide melts. Metall Mater Trans B 41:74–79. https://doi.org/10.1007/s11663-009-9281-8
Jiao S, Zhang L, Zhu H, Fray DJ (2010) Production of NiTi shape memory alloys via electro-deoxidation utilizing an inert anode. Electrochim Acta 55:7016–7020. https://doi.org/10.1016/j.electacta.2010.06.033
Hu L, Song Y, Ge J, Jiao S, Cheng J (2016) Electrochemical metallurgy in CaCl2 -CaO melts on the basis of TiO2-RuO2 inert anode. J Electrochem Soc 163:E33–E38. https://doi.org/10.1149/2.0131603jes
Du Y, Kou M, Tu J, Wang M, Jiao S (2021) An investigation into the anodic behavior of TiB2 in a CaCl2-based molten salt. Corros Sci 178:1–7. https://doi.org/10.1016/j.corsci.2020.109089
Kvalheim E, Haarberg GM, Martinez AM, Jahren HM (2009) Inert anodes for oxygen evolution in molten salts. ECS Trans 16:367–374. https://doi.org/10.1149/1.3159341
Burheim O, Haarberg GM (2010) Effects of inert anodes in the FFC Cambridge reduction of hematite. Trans Inst Miner Metall Sect C. https://doi.org/10.1179/037195510X12665949176454
Yin H, Gao L, Zhu H, Mao X, Gan F, Wang D (2011) On the development of metallic inert anode for molten CaCl2-CaO System. Electrochim Acta 56:3296–3302. https://doi.org/10.1016/j.electacta.2011.01.026
Snook GA, McGregor K, Urban AJ, Lanyon MR, Donelson R, Pownceby MI (2016) Development of a niobium-doped titania inert anode for titanium electrowinning in molten chloride salts. Faraday Discuss 190:35–52. https://doi.org/10.1039/c5fd00235d
Alzamani M, Jafarzadeh K, Fattah-Alhosseini A (2019) EIS study of oxidation heat-treatment effects on corrosion behavior of Ni10Cu11Fe6Al metallic inert anode inside molten calcium chloride salt. Mater Corros 70:605–611. https://doi.org/10.1002/maco.201810417
Alzamani M, Jafarzadeh K, Fattah-alhosseini A (2021) Development of lanthanum doped Ni10Cu11Fe6Al as a new inert anode in molten salt calcium chloride for titanium oxide electrolysis. J Alloys Compd 876:159997. https://doi.org/10.1016/j.jallcom.2021.159997
Choi EY, Lee JW, Park JJ, Hur JM, Kim JK, Jung KY, Jeong SM (2012) Electrochemical reduction behavior of a highly porous SIMFUEL particle in a LiCl molten salt. Chem Eng J 207–208:514–520. https://doi.org/10.1016/J.CEJ.2012.06.161
Choi EY, Jeong SM (2015) Electrochemical processing of spent nuclear fuels: an overview of oxide reduction in pyroprocessing technology. Prog Natl Sci Mater Int 25:572–582. https://doi.org/10.1016/j.pnsc.2015.11.001
Choi E-Y, Lee J, Heo DH, Lee SK, Jeon MK, Hong SS, Kim S-W, Kang HW, Jeon S-C, Hur J-M (2017) Electrolytic reduction runs of 0.6 kg scale-simulated oxide fuel in a Li2O-LiCl molten salt using metal anode shrouds. J Nucl Mater 489:1–8. https://doi.org/10.1016/j.jnucmat.2017.03.035
Iizuka M, Inoue T, Ougier M, Glatz J-P (2007) Electrochemical reduction of (U, Pu)O2 in molten LiCl and CaCl2 electrolytes. J Nucl Sci Technol 44:801–813. https://doi.org/10.1080/18811248.2007.9711869
Jeong SM, Shin H-S, Cho S-H, Hur J-M, Lee HS (2009) Electrochemical behavior of a platinum anode for reduction of uranium oxide in a LiCl molten salt. Electrochim Acta 54:6335–6340. https://doi.org/10.1016/j.electacta.2009.05.080
Joseph TB, Sanil N, Shakila L, Mohandas KS, Nagarajan K (2014) A cyclic voltammetry study of the electrochemical behavior of platinum in oxide-ion rich LiCl melts. Electrochim Acta 139:394–400. https://doi.org/10.1016/j.electacta.2014.07.025
Sakamura Y, Kurata M, Inoue T (2006) Electrochemical reduction of UO2 in molten CaCl2 or LiCl. J Electrochem Soc 153:D31–D31. https://doi.org/10.1149/1.2160430
Pertuiset J, Gibilaro M, Lemoine O, Chamelot P, Bourges G, Massot L (2023) Electrochemical behavior of gold, palladium and platinum as inert anode materials for molten chloride electrolysis. Electrochim Acta 439:141598. https://doi.org/10.1016/J.ELECTACTA.2022.141598
Mukherjee A, Kumaresan R, Ghosh S (2021) Redox behaviour of CaCl2 melts in presence of moisture as impurity. Part I: cyclic voltammetry. J Electroanal Chem 902:5778. https://doi.org/10.1016/j.jelechem.2021.115778
Mukherjee A, Kumaresan R, Ghosh S (2021) Redox behaviour of CaCl2 melts in presence of moisture as impurity. Part II: electrochemical impedance spectroscopy. J Electroanal Chem 901:5710. https://doi.org/10.1016/j.jelechem.2021.115710
Roine A (2008) HSC Chemistry (Version 5.11), Outokumpu Research Oy, Finland
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, Hack K, Jung IH, Kang YB, Melançon J, Pelton AD, Petersen S, Robelin C, Sangster J, Spencer P, Van Ende MA (2016) Reprint of: FactSage thermochemical software and databases, 2010–2016. Calphad 55:1–19. https://doi.org/10.1016/J.CALPHAD.2016.07.004
Sauzet H, Collet R, Héau C, Pupier C, Rodrigues D, Cannes C, Delpech S (2022) Redox properties of the carbonate molten salt Li2CO3-Na2CO3-K2CO3. Electrochim Acta 405:139765. https://doi.org/10.1016/J.ELECTACTA.2021.139765
Yao B, Xiao Y, Jia Y, Wu S, Yang M, He H (2022) Diffusion behavior of oxygen in the electro-deoxidation of uranium oxide in LiCl-rich melt. J Nucl Mater 562:153582. https://doi.org/10.1016/J.JNUCMAT.2022.153582
Descallar-Arriesgado RF, Kobayashi N, Kikuchi T, Suzuki RO (2011) Calciothermic reduction of NiO by molten salt electrolysis of CaO in CaCl2 melt. Electrochim Acta 56:8422–8429. https://doi.org/10.1016/j.electacta.2011.07.027
Acknowledgements
The authors wish to thank Dr. R. Raja Madhavan for carrying out the XRD analyses and Dr. Pradyumna Kumar Parida and Dr. S. Amirthapandian for recording the SEM images. The authors acknowledge Mrs. Shakila Logu, Mr. V. Arun Kumar and Mr. Mohd. Sufiyan Khan for their help in conducting some of the molten salt experiments.
Author information
Authors and Affiliations
Contributions
AM: conceptualization, methodology, formal analysis and investigation, writing–original draft preparation; RK: methodology, formal analysis, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukherjee, A., Kumaresan, R. Investigation on the application of platinum anode towards direct electrochemical reduction of solid metal oxides in CaCl2–CaO melt. J Appl Electrochem (2024). https://doi.org/10.1007/s10800-024-02096-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10800-024-02096-x