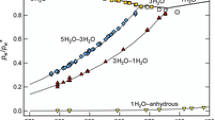
Abstract
Solubility in the ternary system CuCl-NH4Cl-H2O at 25°C was determined by the method of isothermal lifting of oversaturation. A comparative analysis of solubility in this system and the previously studied systems CuCl-MCl-H2O (M+ = Li+, Na+, K+, Cs+) was made. The results obtained were interpreted in terms of competition between hydration, association, and complexation processes in water-salt systems.
Similar content being viewed by others
References
Barachevskii, V.A., Lashkov, G.I., and Tsekhomskii, V.A., Fotokhromizm i ego primenenie (Photochromism and Its Application), Moscow: Khimiya, 1977, pp. 74–161.
Zimin, L.G., Gaponenko, S.V., Lebed, V.Ju., et al., J. Modern Opt., 1990, vol. 37, no. 5, pp. 849–853.
Valov, P.M., Gracheva, L.V., and Leiman, V.I., Fiz. Khim. Stekla, 1997, vol. 23, no. 2, pp. 187–198.
Golubkov, V.V., Vasil’ev, M.I., Pshenitsyna, V.V., and Tsekhomskii, V.A., Fiz. Khim. Stekla, 2000, vol. 26, no. 1, pp. 48–54.
Chernykh, L.V., Tsekhomskii, V.A., and Skripkin, M.Yu., Trudy I Mezhdunarodnoi konferentsii “Khimiya vysokoorganizovannykh veshchestv i nauchnye osnovy nanotekhnologii” (Proc. I Int. Conf. “Chemistry of Highly Organized Substances and Scientific Foundations of Nanotechnology”), St. Petersburg, 1996, vol. 3, pp. 558–561.
Ksandrov, N.V., Nikandrov, I.S., and Bochkova, K.F., Zh. Prikl. Khim., 1994, vol. 67, no. 6, pp. 982–986.
Ksandrov, N.V., Nikandrov, I.S., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 1995, vol. 38, no. 3, pp. 53–59.
Ksandrov, N.V., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 1999, vol. 42, no. 4, pp. 146–148.
Sladkov, A.M. and Gol’ding, I.R., Usp. Khim., 1979, vol. 10, no. 9, pp. 1625–1679.
Temkin, O.N., Flid, R.M., Shestakov, G.K., et al., Kinet. Katal., 1969, vol. 10, no. 6, pp. 1230–1238.
Han, X., Stoltz, V.M., and Corey, E.J., J. Am. Chem. Soc., 1999, vol. 121, no. 33, pp. 7600–7605.
Shestakov, G.K., Tikhonov, G.F., Temkin, O.N., and Flid, R.M., Kinet. Katal., 1970, vol. 11, no. 4, pp. 875–881.
Donkervoort, J.G., Vicario, J.L., Jastrzebski, J.T.B.H., et al., J. Organomet. Chem., 1998, vol. 558, nos. 1–2, pp. 61–69.
Tanaka, H, Yamaguchi, Y., Sumida, S.-I., and Torii, S.H., Chem. Commun., 1996, no. 24, pp. 2705–2706.
Tanaka, H, Yamaguchi, Y., Sumida, S.-I., and Torii, S.H., J. Chem. Soc., Perkin Trans. 1, 1999, no. 23, pp. 3463–3468.
Chernykh, L.V. and Skripkin, M.Yu., Zh. Obshch. Khim., 1996, vol. 66, no. 1, pp. 26–30.
Stepakova, L.V. and Skripkin, M.Yu., Zh. Neorg. Khim., 1999, vol. 44, no. 2, pp. 325–326.
Skripkin, M.Yu., Chernykh, L.V., Stepakova, L.V., and Samokhvalova, V.A., Zh. Prikl. Khim., 2002, vol. 75, no. 7, pp. 1069–1071.
Morozova, I.S. and Ustavshchikova, G.V., Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, 1943, no. 1, pp. 74–80.
Sillen, L.G. and Martell, A.E., Stability Constants of Metal-Ion Complexes: Section 1. Inorganic Ligands, London: Pergamon, 1971, pp. 163–168.
Kumok, V.N., Kuleshova, O.N., and Karabin, L.A., Proizvedeniya rastvorimosti (Solubility Products), Novosibirsk: Nauka, 1983.
Solubility Data Series: vol. 46. Copper(I) Halides and Pseudohalides, Fritz, J.J. and Koninsberger, A., Eds., Oxford: Pergamon, 1997.
Chernykh, L.V. and Skripkin, M.Yu., Koord. Khim., 1996, vol. 22, no. 5, pp. 413–416
Grinberg, A.A., Vvedenie v khimiyu kompleksnykh soedinenii (Introduction to the Chemistry of Complex Compounds), Moscow: Khimiya, 1951, pp. 378–382.
Skripkin, M.Yu. and Chernykh, L.V., Zh. Neorg. Khim., 1994, vol. 39, no. 7, pp. 1747–1751.
Brink, C., Acta Cryst., 1949, vol. 2, pp. 158–163.
Brink, C. and van Arkel, A.E, Acta Cryst., 1952, vol. 5, pp. 506–510.
Brink, C., Binnendijk, N.F., and van de Linde, J., Acta Cryst., 1954, vol. 7, pp. 176–180.
Meyer, G., Z. Anorg. Allg. Chem., 1984, vol. 515, pp. 127–132.
Author information
Authors and Affiliations
Additional information
Original Russian Text © L. V. Stepakova, M. Yu. Skripkin, L. V. Chernykh, 2006, published in Zhurnal Prikladnoi Khimii, 2006, Vol. 79, No. 4, pp. 560–564.
Rights and permissions
About this article
Cite this article
Stepakova, L.V., Skripkin, M.Y. & Chernykh, L.V. Comparative analysis of solubility in CuCl-MCl-H2O systems at 25°C (M+ = Li+, Cs+, NH +4 ). Russ J Appl Chem 79, 549–554 (2006). https://doi.org/10.1134/S1070427206040082
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070427206040082