Abstract
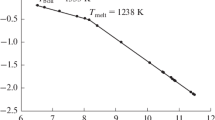
The saturated and unsaturated vapor pressures of tris(2,2,6,6-tetramethylheptane-3,5-dionato)yttrium(III), Y(thd)3, were measured by the static method with a membrane null-manometer in wide ranges of temperature (179.5–418°C) and pressure (300–5220 Pa). The average molecular weight of the compound in unsaturated vapor calculated from the obtained data unambiguously indicates that the gas phase above the liquid compound consists of the Y2(thd)6 dimer molecules. It follows from the temperature dependence of the molecular weight that in the unsaturated vapor the dimer dissociates into the monomers Y(thd)3, and at a higher temperature, the monomer dissociates to form Y(thd)2. Thermodynamic characteristics of the dissociation processes were calculated, and the thermodynamic parameters of the Y(thd)3 evaporation were refined to give the results, which agree well with the mass spectrometry and electron diffraction data for the Y(thd)3 vapor.





Similar content being viewed by others
REFERENCES
MacManus-Driscoll, J.L., Annu. Rev. Mater. Sci., 1998, vol. 28, p. 421.
CRC Hand Book of Chemistry and Physics, Lide, D.R., Ed., Boca Raton: CRC Press, 2008–2009.
Harris, D.C., Infrared Phys. Technol., 1998, vol. 39, no. 4, p. 185.
Swamy, V., Dubrovinskaya, N.A., and Dubrovinsky, L.S., J. Mater. Res., 1999, vol. 14, no. 2, p. 456.
Igumenov, I.K. and Aksenov, A.N., Therm. Eng., 2017, vol. 64, p. 865. https://doi.org/10.1134/S0040601517120035
Tu, R. and Goto, T., Mater Trans., 2005, vol. 46, p. 1318.
Vargas Garcia, J.R. and Goto, T., Sci. Technol. Adv. Mater., 2003, vol. 4, p. 397. https://doi.org/10.1016/S1468-6996(03)00048-2
Wahl, G., Nemetz, W., Giannozzi, M., Rushworth, S., Baxter, D., Archer, N., Cernuschi, F., and Boyle, N., J. Eng. Gas Turb. Power., 2000, vol. 123, p. 520.
Zelenina, L.N., Chusova, T.P., Zherikova, K.V., Nazarova, A.A., and Igumenov, I.K., J. Thermal Anal. Calorim., 2018, vol. 133, p. 1157. https://doi.org/10.1007/s10973-018-7241-8
Girichev, G.V., Giricheva, N.I., Belova, N.V., Kaul’, A.R., Kuz’mina, N.P., and Gorbenko, O.Yu., Zh. Neorg. Khim., 1993, vol. 38, no. 2, p. 342.
Belova, N.V., Giricheva, N.I., Girichev, G.V., Shlykov, S.A., Kharlanova, E.V., Kuzmina, N.P., and Kaul, A.R., J. Str. Chem., 1997, vol. 38, no. 3, p. 395.
Titov, V.A. and Kokovin, G.A., Matematicheskiye metody v khimicheskoy termodinamike (Mathematical Methods in Chemical Thermodynamics), Novosibirsk: Nauka, 1980, p. 98.
Zel’dovich, Ya.B., Zh. Fiz. Khim., 1938, vol. 11, no. 5, p. 685.
Gromilov, S.A., Baidina, I.A., Prokhorova, S.A., and Stabnikov, P.A., Zh. Strukt. Khim., 1995, vol. 36, no. 3, p. 559.
Zherikova, K.V., Zelenina, L.N., Chusova, T.P., Gelfond, N.V., and Morozova, N.B., J. Chem. Thermodyn., 2016, vol. 101, p. 162. https://doi.org/10.1016/j.jct.2016.05.020
Suvorov, A.V., Termodinamicheskaya khimiya paroobraznogo sostoyaniya (Thermodynamic Chemistry of the Vapor State), Leningrad: Khimiya, 1970, p. 46.
Zelenina, L.N., Titov, V.A., Chusova, T.P., Stenin, Yu.G., and Titov, A.A., J. Chem. Thermodyn., 2003, vol. 35, p. 1601. https://doi.org/10.1016/S0021-9614(03)00123-X
Zelenina, L.N., Chusova, T.P., and Vasilyeva, I.G., J. Chem. Thermodyn., 2013, vol. 57, p. 101. https://doi.org/10.1016/j.jct.2012.08.005
ACKNOWLEDGMENTS
The authors are grateful to K.V. Zherikova (Institute of Inorganic Chemistry SB RAS) for the synthesis of Y(thd)3 samples.
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of a state assignment (no. NIOKTR AAAA-A17-117040610358-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 10, pp. 1541–1547 https://doi.org/10.31857/S0044460X21100097.
To the 90th Anniversary of A.V. Suvorov
Rights and permissions
About this article
Cite this article
Zelenina, L.N., Chusova, T.P. Tensimetric Study of the Tris(2,2,6,6-tetramethylheptan-3,5-dionato)yttrium(III) Dissociation in a Gas Phase. Russ J Gen Chem 91, 1984–1989 (2021). https://doi.org/10.1134/S1070363221100091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221100091