Abstract
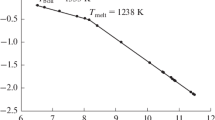
Treatment of the literature data on the vapor pressure of yttrium trifluoride using the new thermodynamic functions of YF3 molecules gave a refined value of the sublimation enthalpy of yttrium trifluoride in the form of a monomer and the enthalpy of formation of YF3(g). The quantum-chemical calculations of YF3 and Y2F6 molecules were performed, and the energy of dissociation of the dimer molecules into two monomer molecules was calculated. Using these data, the enthalpy of sublimation of yttrium trifluoride in the form of a dimer was found, and the formation enthalpy of Y2F6(g) was calculated. The composition of the yttrium trifluoride vapor was calculated: the pressure ratio of Y2F6 and YF3, Pd/Pm, in the range 1400–3000 K increased from ≈2 × 10–4 to ≈2 × 10–2. The obtained thermochemical values were entered into the database of IVTANTERMO software.

Similar content being viewed by others
REFERENCES
E. L. Osina and A. V. Gusarov, High Temp. 53, 817 (2015).
E. L. Osina and L. N. Gorokhov, High Temp. 55, 615 (2017).
D. L. Hildenbrand and K. H. Lau, J. Chem. Phys. 102, 3769 (1995).
E. L. Osina and D. M. Kovtun, Russ. J. Phys. Chem. A 92 (5), 836 (2018).
G. V. Belov, V. S. Iorish, and V. S. Yungman, High Temp. 38, 191 (2000).
E. Rudzitis, H. M. Feder, and W. N. Hubbard, J. Phys. Chem. 69, 2305 (1965).
H. E. Flotow and P. A. G. O’Hare, J. Chem. Phys. 74, 3046 (1981).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 03 Revision B.03 (Gaussian Inc., Pittsburgh, PA, 2003).
K. A. Peterson, D. Figgen, M. Dolg, and H. Stoll, J. Chem. Phys. 126, 124101 (2007).
www.tc.uni-koeln.de/PP/index.en.html.
T. H. Dunning, Jr., J. Chem. Phys. 90, 1007 (1989).
G. Jansen and B. A. Hess, Phys. Rev. A 39, 6016 (1989).
S. Simon, M. Duran, and J. J. Dannenberg, J. Chem. Phys. 105, 11024 (1996).
X. Wang and L. Andrews, J. Phys. Chem. A 114, 2293 (2010).
H. B. Skinner and A. W. Searcy, J. Phys. Chem. 76, 108 (1971).
ACKNOWLEDGMENTS
This study was financially supported by the Russian Scientific Foundation (grant no. 14-50-00124). Some part of the quantum-chemical calculations were performed by D.M. Kovtun (Faculty of Chemistry, Moscow State University).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Gorokhov, L.N., Osina, E.L. & Kovtun, D.M. Thermodynamics of Evaporation of Yttrium Trifluoride in the Form of YF3 and Y2F6. Russ. J. Phys. Chem. 92, 2104–2106 (2018). https://doi.org/10.1134/S0036024418110122
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418110122