Abstract
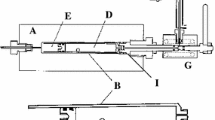
The L-tryptophan sublimation was studied by high-temperature mass spectrometry in the temperature range 395–493 K. The compound evaporates congruently in the form of monomeric molecules. The saturated vapor pressure at 485 K was determined by the Knudsen effusion method. In combination with mass spectrometric data, the pressure equation ln (p, Pa) = –(19943±304)/T + (40.568±0.688) is recommended for the temperature range 395–493 K. The enthalpy of 167.5±1.6 kJ/mol for sublimation at 298.15 K was determined on the basis of the second and third laws of thermodynamics.



Similar content being viewed by others
REFERENCES
Svec, H.J. and Clyde, D.D., J. Chem. Eng. Data, 1965, vol. 10, no. 2, p. 151. https://doi.org/10.1021/je60025a024
de Kruif, C.G., Voogd, J., and Offringa, J.C.A., J. Chem. Thermodyn., 1979, vol. 11, no. 7, p. 651. https://doi.org/10.1016/0021-9614(79)90030-2
Sabbah, R. and Minadakis, C., Thermochim. Acta, 1981, vol. 43, no. 3, p. 269. https://doi.org/10.1016/0040-6031(81)85184-2
Tyunina, V.V., Krasnov, A.V., Tyunina, E.Yu., Badelin, V.G., and Girichev, G.V., J. Chem. Thermodyn., 2014, vol. 74, p. 221. https://doi.org/10.1016/j.jct.2014.02.003
Tyunina, V.V., Krasnov, A.V., Tyunina, E.Yu., Badelin, V.G., and Rybkin, V.V., J. Chem. Thermodyn., 2019, vol. 135, p. 287. https://doi.org/10.1016/j.jct.2019.04.006
Štejfa, V., Pokorný, V., Miranda, C.F.P., Fernandes, Ó.O.P., and Santos, L.M.N.B.F., ChemPhysChem., 2020, vol. 21, no. 9, p. 938. https://doi.org/10.1002/cphc.202000078
Drowart, J., Chatillon, C., Hastie, J., and Bonnell, D., Pure Appl. Chem., 2005, vol. 77, no. 4, p. 683. https://doi.org/10.1351/pac200577040683
Sainio, E.-L., Pulkki, K., and Young, S.N., Amino Acids, 1996, vol. 10, no. 1, p. 21. https://doi.org/10.1007/BF00806091
Richard, D.M., Dawes, M.A., Mathias, C.W., Acheson, A., Hill-Kapturczak, N., and Dougherty, D.M., Int. J. Tryptophan Res., 2009, vol. 2, p. 45. https://doi.org/10.4137/ijtr.s2129
Wright, L.R. and Borkman, R.F., J. Am. Chem. Soc., 1980, vol. 102, no. 20, p. 6207. https://doi.org/10.1021/ja00540a006
Kishor, Sh., Dhayal, S., Mathur, M., and Ramaniah, L.M., Mol. Phys., 2008, vol. 106, no. 19, p. 2289. https://doi.org/10.1080/00268970802422577
Close, D.M., J. Phys. Chem. A, 2011, vol. 115, no. 13, p. 2900. https://doi.org/10.1021/jp200503z
Dehareng, D. and Dive, G., Int. J. Mol. Sci., 2004, vol. 5, no. 11, p. 301. https://doi.org/10.3390/i5110301
Ramaniah, L.M., Chakrabarti, A., Kshirsagar, R.J., Kamal, C., and Banerjee, A., Mol. Phys., 2011, vol. 109, no. 6, p. 875. https://doi.org/10.1080/00268976.2011.558027
Baek, K.Y., Fujimura, Y., Hayash, M., Lin, S.H., and Kim, S.K., J. Phys. Chem. A, 2011, vol. 115, no. 34, p. 9658. https://doi.org/10.1021/jp200826z
Kaczor, A., Reva, I.D., Proniewicz, L.M., and Fausto, R., J. Phys. Chem. A, 2007, vol. 111, no. 15, p. 2957. https://doi.org/10.1021/jp070097c
Sanz, M.E., Cabezas, C., Mata, S., and Alonso, J.L., J. Chem. Phys., 2014, vol. 140, no. 20, p. 204308. https://doi.org/10.1063/1.4876001
Yuan, Y., Mills, M.J.L., Popelier, P.L.A., and Jensen, F., J. Phys. Chem. A, 2014, vol. 118, no. 36, p. 7876. https://doi.org/10.1021/jp503460m
Krauklis, I.V., Tulub, A.V., and Shtyrov, A.A., J. Struct. Chem., 2017, vol. 58, no. 7, p. 1263. https://doi.org/10.1134/S0022476617070010
Dunaeva, V.V., Girichev, G.V., and Giricheva, N.I., ChemChemTech., 2020, vol. 63, no. 3, p. 37. https://doi.org/10.6060/ivkkt.20206303.6112
Chao, J., Hall, K.R., Marsh, K.N., and Wilhoit, R.C., J. Phys. Chem. Ref. Data, 1986, vol. 15, no. 4, p. 1369. https://doi.org/10.1063/1.555769
Lide, D.R., CRC Handbook of Chemistry and Physics, Boca Raton: CRC Press, 2007, p. 3.
NIST Chemistry WebBook, NIST Standard Reference Database Number 69, Linstrom, P.J. an Mallard, W.G., Eds., National Institute of Standards and Technology, Gaithersburg MD, 20899. https://doi.org/10.18434/T4D303
Wilson, K.R., Jimenez-Cruz, M., Nicolas, C., Belau, L., Leone, S.R., and Ahmed, M., J. Phys. Chem. A, 2006, vol. 110, no. 6, p. 2106. https://doi.org/10.1021/jp0543734
Plekan, O., Feyer, V., Richter, R., Coreno, M., and Prince, K.C., Mol. Phys., 2008, vol. 106, nos. 9–10, p. 1143. https://doi.org/10.1080/00268970801974875
Belov, G.V., Iorish, V.S., and Yungman, V.S., CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 1999, vol. 23, no. 2, p. 173. https://doi.org/10.1016/S0364-5916(99)00023-1
Lukyanova, V.A., Druzhinina, A.I., Pimenova, S.M., Ioutsi, V.A., Buyanovskaya, A.G., Takazova, R.U., Sagadeyev, E.V., and Gimadeev, A.A., J. Chem. Thermodyn., 2017, vol. 105, p. 44. https://doi.org/10.1016/j.jct.2016.09.041
Dorofeeva, O.V. and Ryzhova, O.N., J. Phys. Chem. A, 2014, vol. 118, no. 19, p. 3490. https://doi.org/10.1021/jp501357y
Kim, K. and Jordan, K.D., J. Phys. Chem., 1994, vol. 98, no. 40, p. 10089. https://doi.org/10.1021/j100091a024
Stephens, P.J., Devlin, F.J., Chabalowski, C.F., and Frisch, M.J., J. Phys. Chem., 1994, vol. 98, no. 45, p. 11623. https://doi.org/10.1021/j100096a001
Dunning, T.H. Jr., J. Chem. Phys., 1989, vol. 90, p. 1007. https://doi.org/10.1063/1.456153
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr., J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul,A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford CT, 2004.
Pogrebnoi, A.M., Kudin, L.S., Kuznetsov, A.Yu., and Butman, M.F., Rapid Commun. Mass Spectrom., 1997, vol. 11, no. 14, p. 1536. https://doi.org/10.1002/(SICI)1097-0231(199709)11:14%3C1536::AID-RCM31%3E3.0.CO;2-D
ACKNOWLEDGMENTS
The authors are grateful to E.V. Tyunina (G.A. Krestov Institute of Solution Chemistry, Russian Academy of Sciences) for providing the L-tryptophan preparation and to N.A. Ermolaeva and L.B. Smirnova (Center for Collective Use of the Ivanovo State University of Chemistry and Technology) for recording IR spectra and performing thermogravimetric analysis of samples.
Funding
The scientific research was carried out with the support of the Ivanovo State University of Chemistry and Technology (grant no. 04-ISUCT/1-21).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 10, pp. 1490–1498 https://doi.org/10.31857/S0044460X21100036.
To the 90th Anniversary of A.V. Suvorov
Supplementary information
Rights and permissions
About this article
Cite this article
Motalov, V.B., Korobov, M.A., Dunaev, A.M. et al. Vapor Pressure and Thermodynamics of L-Tryptophan Sublimation. Russ J Gen Chem 91, 1938–1945 (2021). https://doi.org/10.1134/S1070363221100030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221100030