Abstract
The reaction of 2-thioxoazines with chlorokojic acid in the presence of KOH in DMF led to the formation of new hybrid molecules containing fragments of kojic acid and azaheterocycle linked by the SCH2 spacer. In silico prediction of bioavailability parameters was carried out, possible protein targets were predicted by the protein ligand docking method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Kojic acid 1 (5-hydroxy-2-hydroxymethyl-4H-pyran-4-one) is widely used in the pharmaceutical industry, agrochemistry, cosmetology [1–5], and as a ligand for complex compounds [6] and building blocks for construction of biologically active heterocyclic molecules [7–9]. Being one of the most studied and available non-toxic tyrosinase inhibitors [10, 11], kojic acid as a bioactive molecule is not devoid of disadvantages, among which should be noted the lack of stability during storage and a relatively low inhibitory activity. For this reason, in recent years, the direction of the γ-pyrones chemistry devoted to the preparation of conjugates of kojic acid or other derivatives functionalized at C6OH groups has been developed [12–18]. Among the new effective tyrosinase inhibitors, it is worth noting a number of hybrid molecules combining a kojic acid residue and an aryl/hetaryl moiety – for example, Mannich base 2 [18], 1,2,3-triazole derivatives 3 [19, 20], substituted thiophenol 4 [21] or 4-amino-1,2,4-triazoles 5 [22] (Scheme 1). In addition, a number of hybrid structures with a kojic acid motif are known, demonstrating a different spectrum of biological action. Thus, functionalization with a kojic acid was used to create 10B-labeled derivatives of dodecaboranethiol 6 for boron neutron capture therapy of cancer [23]. Compound ML221 7 is a highly effective antagonist of the APJ apelin receptor with possible use in the therapy of cardiovascular diseases [24] (Scheme 1). Pivalic acid esters 8 have been shown to be effective inhibitors of neutrophil elastase suitable for the treatment of inflammatory lung diseases [25], while piperazine derivatives 9 exhibit anti-tuberculosis [26] and anticancer [27] effects. According to patent data [28], 2-mercaptoimidazoline derivatives 10 exhibit antibacterial activity.
One of the most accessible methods of functionalization of kojic acid is its transformation into bromo- or chlorokojic acid 11, with further replacement of the halogen atom by various nucleophiles such as thioureas [28–33], alkyl mercaptans [29, 30, 34], thiophenols [21, 35], sodium arylsulfinates [36], alkali metal thiocyanates [36–39], S-glycosylisothiuronium salts [40], various mercaptoazoles [22, 25, 28, 29, 41], 2-mercaptopyrimidines [24, 42], 2-mercaptoquinazolines [43, 44], mono- and dithiophosphates [45, 46]. Many of the compounds obtained in this way are of interest for agrochemistry as growth regulators and herbicides comparable in efficiency to Fluazifop-P [29], or as insecticides [45, 46]. In general, the analysis of literature data allows us to conclude that the reaction of halokojic acids with heterocyclic S-nucleophiles has been relatively little studied. For example, the reaction of 5-hydroxy-2-chloromethyl-4H-pyran-4-one with available 3-cyanopyridine-2(1H)-thiones and tautomeric mercaptans (for review works, see [47–54]) widely used in heterocyclic synthesis have not been reported earlier. At the same time, the target products can be of interest as promising pharmaceuticals, agrochemicals and reagents for fine organic synthesis.
We performed the reaction of some active S-nucleophiles of the azaheterocyclic series with chlorkojic acid 11 (Hlg = Cl). It was found that 2-thioxopyridines 12а, 12b react with chloride 11 in the presence of 1 equiv. of 10% aqueous KOH with the formation of previously unknown hybrid molecules 13а, 13b containing kojic acid and nicotinonitrile fragments (Scheme 2). Under similar conditions, 2-thioxo-1,2-dihydroquinoxaline 14 was converted to compound 15 in 28% yield.
Structure of the obtained compounds was confirmed by a complex of spectral data. The IR spectra of compounds 13 and 15 show an absorption band at 1646–1649 cm–1, corresponding to stretching vibrations of the conjugated carbonyl group of γ-pyrone, as well as a broad band at 3226–3253 cm–1 (O–H). The spectra of compounds 13а, 13b also show an absorption band in the range of 2218–2223 cm–1 (conjugated cyano group). The 1Н NMR spectra of compounds 13 and 15 exhibit singlets at 4.47–4.64 ppm (SCH2), as well as signals of the 5-hydroxypyran-4-one fragment at 6.47–6.59 (Н3), 8.01–8.09 (Н6), and 9.11– 9.15 ppm (OH). The 13C NMR spectra of compounds 13 and 15 contain characteristic signals at 30.8–31.6 (SCH2), 112.1–112.9 (С3-pyran), 139.6–139.8 (С6-pyran), 143.5–145.8 (С5-pyran), 163.1–163.8 (C2-pyran), and 173.6 ppm (C=O).
In the context of the known biological activity of kojic acid derivatives [7–11], nicotinonitriles, and quinoxalines (for recent reviews see [55–58] and [59–67], respectively), it seemed appropriate to perform a preliminary in silico study of possible targets, ADMET parameters, and the bioavailability criteria for new hybrid molecules. Analysis of the structures for compliance with the “Lipinski’s rule of five” [molecule mass (MW) ≤ 500, сLogP ≤ 5.0, TPSA ≤ 140 Å2, number of hydrogen bond acceptors ≤ 10, donors ≤ 5] [68–70] was carried out using the OSIRIS Property Explorer software service [71]. The following parameters were calculated: сLog P (logarithm of the partition coefficient between n-octanol and water log (coctanol/cwater), solubility (log S), Topological Polar Surface Area (TPSA), a number of toxicological characteristics such as risks of side effects (mutagenic, oncogenic, reproductive effects), the drug-likeness parameter, as well as the overall assessment of the pharmacological potential of the compound (drug score). The calculated data are presented in Table 1.
The cLog P value for all the studied structures indicates probable good absorption and permeability [68–70]. At the same time, for all the compounds, the log S value < –4.0 indicates low solubility (less than 1×10–4 mol/L). The molecular weights of all the compounds and the TPSA parameter met the criteria for oral bioavailability. The studied compounds show a moderate risk of oncogenic action associated with the presence of a 5-hydroxypyran-4-one fragment. However, the total predicted values of the drug score are rather high. To predict the ADMET parameters (Absorption, Distribution, Metabolism, Excretion, Toxicity) and probable targets, the SwissADME [72] and GUSAR [73] software packages were also used. The results are shown in Table 2. In general, the assessment of acute toxicity allows all the studied compounds to be classified as IV and V hazard classes according to the OECD criteria [74]. For these compounds, an inhibitory effect on a wide range of cytochrome P450 isoforms is postulated.
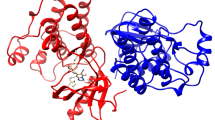
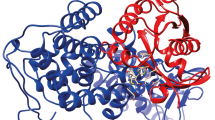
Possible protein targets for the obtained compounds were predicted using the new Galaxy Sagittarius protein ligand docking protocol [75] based on the GalaxyWeb web server [76, 77]. The 3D joint structures were pre-optimized by molecular mechanics in an MM2 force field to optimize geometry and minimize energy. Docking using the GalaxySagittarius protocol was performed in the Binding compatability prediction and Re-ranking using docking modes. Table 3 shows the results of docking for each of compounds 13а, 13b, 15 for 10 target-ligand complexes with the minimum free binding energy ΔGbind and the best estimate of the protein-ligand interaction. Predicted protein targets are specified using ID-identifiers in the Protein Data Bank (PDB) and in the UniProt database. As one can see from the Table 3, common receptors for compounds 13a, 13b, and 15 are RPA (Replication Protein A, PDB ID 4luv) phosphoprotein responsible for DNA replication and repair in eukaryotes, 3-phosphoinositol-dependent protein kinase-1 (PDK1, PDB ID 4rqv), and apoptosis regulator Mcl-1 (PDB ID 6qfq) (Fig. 1). Thus, 5-hydroxy-2-[(hetarylthio)methyl]-4H-pyran-4-ones 13а, 13b, and 15 can be considered as promising objects for screening in order to search for new agents for the treatment and therapy of oncological diseases.
Predicted structures of protein-ligand complexes for compound 13а and phosphoprotein RPA (PDB ID 4luv) (a), compound 13а and protein Mcl-1 (PDB ID 6qfq) (b), compound 13b and protein kinase PDK1 (PDB ID 4rqv) (c), quinoxaline 15 and phosphoprotein RPA (PDB ID 4luv) (d) (obtained using the Galaxy Sagittarius protocol). Molecular graphics were visualized using the UCSF Chimera software package [78, 79].
In conclusion, we developed a convenient method for the preparation of previously unknown 5-hydroxy-2-[(hetarylthio)methyl]-4H-pyran-4-ones by the reaction of 2-thioxonicotinonitrile and 2-thioxo-1,2-dihydroquinoxaline derivatives with 5-hydroxy-2-chloromethyl-4H-pyran-4-one (chlorokojic acid). The results of in silico experiments on the assessment of probable protein targets, toxicity, and bioavailability parameters make it possible to consider the obtained compounds as promising objects for the development of new drugs with antitumor action.
EXPERIMENTAL
IR spectra were registered on a Bruker Vertex 70 spectrometer with an ATR attachment. 1H and 13C NMR spectra were recorded on a Bruker Avance III HD 400 MHz instrument (400.17 and 100.63 MHz, respectively) in a DMSO-d6 solution. Chromato-mass spectra were recorded on a Bruker Customer MicrOTOF instrument in the range of m/z 50–1200, the method of ionization was electrospray (ESI). Elemental analysis was performed on an Elementar vario Micro cube instrument. The individuality of the obtained samples was controlled by TLC on Sorbfil-A plates (LLC Imid, Krasnodar), eluent was acetone–hexane mixture (1 : 1) or ethyl acetate, visualized with iodine vapors or UV light.
The starting 3-cyanopyridine-2(1H)-thiones 12а, 12b [80, 81] and 2-thioxo-1,2-dihydroquinoxaline 14 [82] were obtained according to the known methods. Chlorokojic acid 11 was prepared by treating commercial kojic acid with thionyl chloride [83].
General procedure for the synthesis of 5-hydroxy-2-[(hetarylthio)methyl]-4H-pyran-4-ones 13а, 13b, 15. The appropriate 2-thioxonicotinonitrile 12 or thione 14 (2 mmol) was suspended in 2 mL of DMF, then an aqueous 10% KOH solution (1.03 mL, 2 mmol, d 1.09 g/mL) was added. The resulting suspension was stirred at room temperature until dissolved, then through a paper filter it was added dropwise to a solution of 321 mg (2 mmol) of chlorokojic acid 11 in 0.5 mL of DMF. The resulting mixture was stirred for 30 min. The formed precipitate was filtered off, washed with 50% ethanol, and dried at 60°С.
2-{[(5-Hydroxy-4-oxo-4H-pyran-2-yl)methyl]thio}-4,6-diphenylnicotinonitrile (13a). Yield 75%, pale brown powder. IR spectrum, ν, cm–1: 1647 (С=О), 2218 (C≡N), 3239 (O–H). 1Н NMR spectrum, δ, ppm: 4.64 s (2Н, SCH2), 6.50 s (1Н, Н3pyran), 7.53–7.59 m (6Н, Н-Ph), 7.74–7.77 m (2Н, Н-Ph), 7.96 s (1Н, H5-Py), 8.09 s (1Н, Н6pyran), 8.24–8.26 m (2Н, Н-Ph), 9.15 br. s (1Н, OH). 13С DEPTQ NMR spectrum, δC, ppm: 30.8 (SCH2), 103.1 (С–С≡N), 112.2* (C3Н, pyran), 115.5 (С≡N), 116.9* (C5, Py), 127.6* (2C, СН, Ph), 128.7* (2C, СН, Ph), 128.9* (2C, СН, Ph), 129.0* (2C, СН, Ph), 130.2* (С4Н, Ph), 131.0* (С4Н, Ph), 135.5 (C1, Ph), 136.4 (C1, Ph), 139.8* (C6H, pyran), 145.8 (C5, pyran), 154.5 (Py), 158.1 (Py), 160.6 (Py), 163.8 (C2, pyran), 173.6 (C=O). Hereinafter, an asterisk denotes signals in antiphase. Mass spectrum, m/z (Irel, %): 451.05 [M + K]+ (100), 863.15 [2M + K]+. Found, %: C 69.77; H 4.06; N 6.90. C24H16N2O3S (M 412.46). Calculated, %: C 69.89; H 3.91; N 6.79.
2-{[(5-Hydroxy-4-oxo-4H-pyran-2-yl)methyl]thio}-6-phenyl-4-(2,4-dichlorophenyl)nicotinonitrile (13b). Yield 30%, brown powder. IR spectrum, ν, cm–1: 1649 (С=О), 2223 (C≡N), 3226 (O–H). 1Н NMR spectrum, δ, ppm: 4.64 s (2Н, SCH2), 6.47 s (1Н, Н3pyran), 7.52–7.56 m (5Н, Н-Ar), 7.65–7.66 m (2Н, Н-Ar), 7.90 d (1Н, H-Ar, 4J 0.9 Гц), 7.99 s (1Н, H5-Py), 8.01 s (1Н, Н6pyran), 8.22–8.24 m (2Н, Н-Ar). Signal of the OH group does not appear due to deuterium exchange. 13С DEPTQ NMR spectrum, δC, ppm: 30.8 (SCH2), 104.6 (С–С≡N), 112.1* (C3Н, pyran), 114.5 (С≡N), 117.5* (C5, Py), 127.6* (СH, Ar), 127.9* (СH, Ar), 129.1* (СH, Ar), 131.2* (СH, Ar), 132.3* (СH, Ar), 132.5 (Ar), 133.6 (Ar), 135.4 (Ar), 136.1 (Ar), 139.6* (C6H, pyran), 145.8 (C5, pyran), 151.5 (Py), 158.3 (Py), 160.1 (Py), 163.1 (C2, pyran), 173.6 (C=O). Mass spectrum, m/z (Irel, %): 481.50 [M + Na]+ (100), 985.02 [2M + Na]+. Found, %: C 59.74; H 3.10; N 5.94. C24H14Cl2N2O3S (M 481.35). Calculated, %: C 59.89; H 2.93; N 5.82.
5-Hydroxy-2-({[3-(4-methoxyphenyl)quinoxalin-2-yl]thio}methyl)-4H-pyran-4-one (15). Yield 28%, brown powder. IR spectrum, ν, cm–1: 1646 (С=О), 3253 (O–H). 1Н NMR spectrum, δ, ppm: 3.85 s (3Н, MeO), 4.47 s (2Н, SCH2), 6.59 s (1Н, Н3pyran), 7.10–7.12 m (2Н, Н-Ar), 7.72–8.03 m (8Н, Н-Ar, overlapping with the signal of Н5pyran), 9.11 s (1Н, OH). 13С DEPTQ NMR spectrum, δC, ppm: 31.6 (SCH2), 55.3* (MeO), 112.9* (C3Н, pyran), 113.9* (CH, Ar), 127.1* (CH, Ar), 128.8* (CH, Ar), 129.0* (CH, Ar), 129,1* (CH, Ar), 130.4* (CH, Ar), 130.5* (CH, Ar), 139.1 (Ar), 139.8* (CH, pyran), 143.5* (C5, pyran), 145.7 (Ar), 152.4 (Ar), 152.9 (Ar), 160.6 (Ar), 163.6 (C2, pyran), 173.6 (C=O).. Found, %: C 64.15; H 4.22; N 7.24. C21H16N2O4S (M 392.43). Calculated, %: C 64.27; H 4.11; N 7.14.
REFERENCES
Beélik, A., Adv. Carbohyd. Chem., 1956, vol. 11, p. 145. https://doi.org/10.1016/S0096-5332(08)60118-6
Saeedi, M., Eslamifar, M., and Khezri, K., Biomed. Pharmacother., 2019, vol. 110, p. 582. https://doi.org/10.1016/j.biopha.2018.12.006
Aytemir, M.D. and Karakaya, G., Kojic Acid Derivatives. Medicinal Chemistry and Drug Design, Rijeka: InTech Open Access Publisher, 2012, p. 1. https://doi.org/10.5772/31006
Chaudhary, J., Pathak, A.N., and Lakhawat, S., Ann. Res. Rev. Biol., 2014, p. 3165. https://doi.org/10.9734/ARRB/2014/10643
Mohamad, R., Mohamed, M.S., Suhaili, N., Salleh, M.M., and Ariff, A.B., Biotech. Mol. Biol. Rev., 2010, vol. 5, no. 2, p. 24. https://doi.org/10.5897/BMBR2010.0004
Kandioller, W., Kurzwernhart, A., Hanif, M., Meier, S.M., Henke, H., Keppler, B.K., and Hartinger, C.G., J. Organomet. Chem., 2011, vol. 696, no. 5, p. 999. https://doi.org/10.1016/j.jorganchem.2010.11.010
Zirak, M. and Eftekhari-Sis, B., Turk. J. Chem., 2015, vol. 39, no. 3, p. 439. https://doi.org/10.3906/kim-1502-55
Mohajer, F. and Mohammadi Ziarani, G., Heterocycles, 2021, vol. 102, no. 2, p. 211. https://doi.org/10.3987/REV-20-936
Chaudhary, A., Curr. Org. Chem., 2020, vol. 24, no. 14, p. 1643. https://doi.org/10.2174/1385272824999200622113153
Saruno, R., Kato, F., and Ikeno, T., Agric. Biol. Chem., 1979, vol. 43, no. 6, p. 1337. https://doi.org/10.1271/bbb1961.43.1337
Cabanes, J., Chazarra, S., and Garcia-Carmona, F., J. Pharm. Pharmacol., 1994, vol. 46, no. 12, p. 982. https://doi.org/10.1111/j.2042-7158.1994.tb03253.x
Noh, J.M., Kwak, S.Y., Kim, D.H., and Lee, Y.S., Biopolymers (Pept. Sci.), 2007, vol. 88, no. 2, p. 300. https://doi.org/10.1002/bip.20670
Noh, J.M., Kwak, S.Y., Seo, H.S., Seo, J.H., Kim, B.G., and Lee, Y.S., Bioorg. Med. Chem. Lett., 2009, vol. 19, no. 19, p. 5586. https://doi.org/10.1016/j.bmcl.2009.08.041
Lee, Y.S., Park, J.H., Kim, M.H., Seo, S.H., and Kim, H.J., Arch. Pharm., 2006, vol. 339, no. 3, p. 111. https://doi.org/10.1002/ardp.200500213
Singh, B.K., Park, S.H., Lee, H.B., Goo, Y.A., Kim, H.S., Cho, S.H., Lee, J.H., Ahn, G.W., Kim, J.P., Kang, S.M., and Kim, E.K., Ann. Dermatol., 2016, vol. 28, no. 5, p. 555. https://doi.org/10.5021/ad.2016.28.5.555
Lachowicz, J.I., Nurchi, V.M., Crisponi, G., Pelaez, M.D.G.J., Rescigno, A., Stefanowicz, P., Cal, M., and Szewczuk, Z., J. Inorg. Biochem., 2015, vol. 151, p. 36
Hashemi, S.M. and Emami, S., Pharm. Biomed. Res., 2015, vol. 1, no. 1, p. 1. https://doi.org/10.18869/acadpub.pbr.1.1.1
Karakaya, G., Türe, A., Ercan, A., Öncül, S., and Aytemir, M.D., Bioorg. Chem., 2019, vol. 88, paper 102950. https://doi.org/10.1016/j.bioorg.2019.102950
Ashooriha, M., Khoshneviszadeh, M., Khoshneviszadeh, M., Moradi, S.E., Rafiei, A., Kardan, M., and Emami, S., Bioorg. Chem., 2019, vol. 82, p. 414. https://doi.org/10.1016/j.bioorg.2018.10.069
Ashooriha, M., Khoshneviszadeh, M., Khoshneviszadeh, M., Rafiei, A., Kardan, M., Yazdian-Robati, R., and Emami, S., Eur. J. Med. Chem., 2020, vol. 201, Paper 112480. https://doi.org/10.1016/j.ejmech.2020.112480
Rho, H.S., Baek, H.S., Ahn, S.M., Kim, M.K., Ghimeray, A.K., Cho, D.H., and Hwang, J.S., Bull. Korean Chem. Soc., 2010, vol. 31, no. 8, p. 2375. https://doi.org/10.5012/bkcs.2010.31.8.2375
Xie, W., Zhang, J., Ma, X., Yang, W., Zhou, Y., Tang, X., Zou, Y., Li, H., He, J., Xie, S., Zhao, Y., and Liu, F., Chem. Biol. Drug Des., 2015, vol. 86, no. 5, p. 1087. https://doi.org/10.1111/cbdd.12577
Takeuchi, K., Hattori, Y., Kawabata, S., Futamura, G., Hiramatsu, R., Wanibuchi, M., Tanaka, H., Masunaga, S-i., Ono, K., Miyatake, S.-I., and Kirihata, M., Cells, 2020, vol. 9, no. 6, Paper N 1551. https://doi.org/10.3390/cells9061551
Maloney, P.R., Khan, P., Hedrick, M., Gosalia, P., Milewski, M., Li, L., Roth, G.P., Sergienko, E., Suyama, E., Sugarman, E., Nguyen, K., Mehta, A., Vasile, S., Su, Y., Stonich, D., Nguyen, H., Zeng, F.-Y., Novo, A.M., Vicchiarelli, M., Diwan, J., Chung, T.D.Y., Smith, L.H., and Pinkerton, A.B., Bioorg. Med. Chem. Lett., 2012, vol. 22, no. 21, p. 6656. https://doi.org/10.1016/j.bmcl.2012.08.105
Lucas, S.D., Gonçalves, L.M., Carvalho, L.A.R., Correia, H.F., Da Costa, E.M.R., Guedes, R.A., Moreira, R., and Guedes, R.C., J. Med. Chem., 2013, vol. 56, no. 23, p. 9802. https://doi.org/10.1021/jm4011725
Karakaya, G., Aytemir, M.D., Özçelik, B., and Çalış, Ü., J. Enzyme Inhib. Med. Chem., 2013, vol. 28, no. 3, p. 627. https://doi.org/10.3109/14756366.2012.666538
Karakaya, G., Ercan, A., Öncül, S., and Aytemir, M.D., J. Res. Pharm., 2019, vol. 23, no. 4, p. 596. https://doi.org/10.12991/jrp.2019.167
Krimmel, C., Patent US 2700045, 1955; C. A., 1956, vol. 50. 411.
Veverka, M., Chem. Pap., 1992, vol. 46, no. 3, p. 208.
Kipnis, F., Soloway, H., and Ornfelt, J., J. Am. Chem. Soc., 1948, vol. 70, no. 12, p. 4264. https://doi.org/10.1021/ja01192a080
Krimmel, C., Patent US 2715130, 1955.
Krimmel C., Patent US 2851467, 1958.
White, R.L.Jr., Schwan, T.J., and Alaimo, R.J., J. Heterocycl. Chem., 1980, vol. 17, no. 4, p. 817. https://doi.org/10.1002/jhet.5570170442
Rho, H.S., Ahn, S.M., Yoo, D.S., Kim, M.K., Cho, D.H., and Cho, J.Y., Bioorg. Med. Chem. Lett., 2010, vol. 20, no. 22, p. 6569. https://doi.org/10.1016/j.bmcl.2010.09.042
Rho, H.S., Yoo, D.S., Ahn, S.M., Kim, M.K., Cho, D.H., and Cho, J.Y., Bull. Korean Chem. Soc., 2010, vol. 31, no. 11, p. 3463. https://doi.org/10.5012/bkcs.2010.31.11.3463
Uher, M., Kyselicova, L., Rajniakova, O., Hudecova, D., Bransova, J., and Brtko, J., Chem. Pap., 1997, vol. 51, no. 6B, p. 421.
Rondahl, L., Uher, Μ., and Brtko, J., Heterocycl. Commun., 2003, vol. 9, no. 3, p. 257. https://doi.org/10.1515/HC.2003.9.3.257
Uher, M., Szymońska, J., Korenova, A., and Tomasik, P., Monatsh. Chem., 2000, vol. 131, no. 3, p. 301. https://doi.org/10.1007/s007060070106
Bransova, J., Uher, M., Novotny, L., and Brtko, J., Anticancer Res., 1997, vol. 17, p. 1175.
Wu, Z., Cao, A., Ding, W., Zhu, T., and Shen, P., J. Carbohydr. Chem., 2016, vol. 35, no. 7, p. 355. https://doi.org/10.1080/07328303.2016.1261881
Xie, W., Zhang, H., He, J., Zhang, J., Yu, Q., Luo, C., and Li, S., Bioorg. Med. Chem. Lett., 2017, vol. 27, no. 3, p. 530. https://doi.org/10.1016/j.bmcl.2016.12.027
Raje, M., Hin, N., Duvall, B., Ferraris, D.V., Berry, J.F., Thomas, A.G., Alt, J., Rojas, C., Slusher, B.S., and Tsukamoto, T., Bioorg. Med. Chem. Lett., 2013, vol. 23, no. 13, p. 3910. https://doi.org/10.1016/j.bmcl.2013.04.062
Sherafati, M., Mirzazadeh, R., Barzegari, E., Mohammadi-Khanaposhtani, M., Azizian, H., Asgari, M.S., Hosseini, S., Zabihi, E., Mojtabavi, S., Faramarzi, M.A., Mahdavi, M., Larijani, B., Rastegar, H., Hamedifar, H., and Hajimiri, M.H., Bioorg. Chem., 2021, vol. 109, Paper 104703. https://doi.org/10.1016/j.bioorg.2021.104703
Sepehri, N., Iraji, A., Yavari, A., Asgari, M.S., Zamani, S., Hosseini, S., Bahadorikhalili, S., Pirhadi, S., Larijani, B., Khoshneviszadeh, M., Hamedifar, H., Mahdavi, M., and Khoshneviszadeh, M., Bioorg. Med. Chem., 2021, vol. 36, Paper 116044. https://doi.org/10.1016/j.bmc.2021.116044
Schrader, G., Lorenz, W., Cölin, R., and Schlör, H.-H., Patent US 3232830, 1966; C. A., 1966, vol. 64. 15923.
Metivier, J., Patent US 2752283, 1956.
Litvinov, V.P., Rodinovskaya, L.A., Sharanin, Yu.A., Shestopalov, A.M., and Senning, A., J. Sulfur Chem., 1992, vol. 13, no. 1, p. 1. https://doi.org/10.1080/01961779208048951
Litvinov, V.P., Phosphorus, Sulfur, Silicon, Relat. Elem., 1993, vol. 74, no. 1, p. 139. https://doi.org/10.1080/10426509308038105
Litvinov, V.P., Russ. Chem. Bull., 1998, vol. 47, no. 11, p. 2053. https://doi.org/10.1007/BF02494257
Litvinov, V.P., Krivokolysko, S.G., and Dyachenko, V.D., Chem. Heterocycl. Compd., 1999, vol. 35, no. 5, p. 509. https://doi.org/10.1007/BF02324634
Litvinov, V.P., Dotsenko, V.V., and Krivokolysko, S.G., Russ. Chem. Bull., 2005, vol. 54, no. 4, p. 864. https://doi.org/10.1007/s11172-005-0333-1
Litvinov, V.P., Russ. Chem. Rev., 2006, vol. 75, no. 7, p. 577. https://doi.org/10.1070/RC2006v075n07ABEH003619
Litvinov, V.P., Dotsenko, V.V., and Krivokolysko, S.G., Adv. Heterocycl. Chem., 2007, vol. 93, p. 117. https://doi.org/10.1016/S0065-2725(06)93003-7
Dotsenko, V.V., Buryi, D.S., Lukina, D.Yu., and Krivokolysko, S.G., Russ. Chem. Bull., 2020, vol. 69, no. 10, p. 1829. https://doi.org/10.1007/s11172-020-2969-2
Salem, M.A., Helel, M.H., Gouda, M.A., Ammar, Y.A., and El-Gaby, M.S.A., Synth. Commun., 2018, vol. 48, no. 4, p. 345. https://doi.org/10.1080/00397911.2017.1394468
Gouda, M.A., Attia, E., Helal, M.H., and Salem, M.A., J. Heterocycl. Chem., 2018, vol. 55, no. 10, p. 2224. https://doi.org/10.1002/jhet.3298
Gouda, M.A., Hussein, B.H., Helal, M.H., and Salem, M.A., J. Heterocycl. Chem., 2018, vol. 55, no. 7, p. 1524. https://doi.org/10.1002/jhet.3188
Shamroukh, A.H., Kotb, E.R., Anwar, M.M., and Sharaf, M., Egypt. J. Chem., 2021, vol. 64, no. 8, p. 4509. https://doi.org/10.21608/EJCHEM.2021.64971.3392
Quiliano, M. and Aldana, I., Rev. Virtual Quim., 2013, vol. 5, no. 6, p. 1120. https://doi.org/10.5935/1984-6835.20130081
Mamedov, V.A. and Zhukova, N.A., Progress Heterocycl. Chem., 2012, vol. 24, p. 55. https://doi.org/10.1016/B978-0-08-096807-0.00002-6
Mamedov, V.A. and Zhukova, N.A., Progress Heterocycl. Chem., 2013, vol. 25, p. 1. https://doi.org/10.1016/B978-0-08-099406-2.00001-7
Cheng, G., Sa, W., Cao, C., Guo, L., Hao, H., Liu, Z., Wang, X., and Yuan, Z., Front. Pharmacol., 2016, vol. 7, Paper 64. https://doi.org/10.3389/fphar.2016.00064
González, M. and Cerecetto, H., Exp. Opin. Therap. Pat., 2012, vol. 22, no. 11, p. 1289. https://doi.org/10.1517/13543776.2012.724677
Pereira, J.A., Pessoa, A.M., Cordeiro, M.N.D.S., Fernandes, R., Prudêncio, C., Noronha, J.P., and Vieira, M., Eur. J. Med. Chem., 2015, vol. 97, p. 664. https://doi.org/10.1016/j.ejmech.2014.06.058
Montana, M., Mathias, F., Terme, T., and Vanelle, P., Eur. J. Med. Chem., 2019, vol. 163, p. 136. https://doi.org/10.1016/j.ejmech.2018.11.059
Ajani, O.O., Eur. J. Med. Chem., 2014, vol. 85, p. 688. https://doi.org/10.1016/j.ejmech.2014.08.034
El Newahie, A.M.S., Ismail, N.S.M., Abou El Ella, D.A., and Abouzid, K.A.M., Arch. Pharm., 2016, vol. 349, no. 5, p. 309. https://doi.org/10.1002/ardp.201500468
Lipinski, C.A., Lombardo, F., Dominy, B.W., and Feeney, P.J., Adv. Drug. Delivery Rev., 1997, vol. 23, nos. 1–3, p. 4. https://doi.org/10.1016/S0169-409X(96)00423-1
Lipinski, C.A., Drug Discov. Today: Technologies, 2004, vol. 1, no. 4, p. 337. https://doi.org/10.1016/j.ddtec.2004.11.007
Lipinski, C.A., Lombardo, F., Dominy, B.W., and Feeney, P.J., Adv. Drug. Delivery Rev., 2012, vol. 64, Suppl, p. 4. https://doi.org/10.1016/j.addr.2012.09.019
Sander, T., OSIRIS Property Explorer. Idorsia Pharmaceuticals Ltd, Switzerland. http://www.organic-chemistry.org/prog/peo/
Daina, A., Michielin, O., and Zoete, V., Sci. Rep., 2017, vol. 7, Article N 42717. https://doi.org/10.1038/srep42717
Lagunin, A., Zakharov, A., Filimonov, D., and Poroikov, V., Mol. Inform., 2011, vol. 30, nos. 2–3, p. 241. https://doi.org/10.1002/minf.201000151
OECD Environment, Health and Safety Publications Series on Testing and Assessment no. 24. Guidance Document on Acute oral Toxicity Testing. ENV/JM/MONO(2001)4. OECD, Paris. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2001)4&doclanguage=en
Yang, J., Kwon, S., Bae, S.H., Park, K.M., Yoon, C., Lee, J.H., and Seok, C., J. Chem. Inf. Model., 2020, vol. 60, no. 6, p. 3246. https://doi.org/10.1021/acs.jcim.0c00104
GalaxyWEB. A web server for protein structure prediction, refinement, and related methods. Computational Biology Lab, Department of Chemistry, Seoul National University, S. Korea. http://galaxy.seoklab.org/index.html
Ko, J., Park, H., Heo, L., and Seok, C., Nucleic Acids Res., 2012, vol. 40, no. W1, p. W294. https://doi.org/10.1093/nar/gks493
Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Greenblatt, D.M., Meng, E.C., and Ferrin, T.E., J. Сomput. Chem. 2004, vol. 25, no. 13, p. 1605. https://doi.org/10.1002/jcc.20084
UCSF Chimera. Visualization system for exploratory research and analysis developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, US. https://www.rbvi.ucsf.edu/chimera/
Buryi, D.S., Dotsenko, V.V., Levashov, A.S., Lukina, D.Yu., Strelkov, V.D., Aksenov, N.A., Aksenova, I.V., and Netreba, E.E., Russ. J. Gen. Chem., 2019, vol. 89, no. 5, p. 886. https://doi.org/10.1134/S1070363219050050
Shestopalov, A.M., Nikishin, K.G., Gromova, A.V., and Rodinovskaya, L.A., Russ. Chem. Bull., 2003, vol. 52, no. 10, p. 2203. https://doi.org/10.1023/B:RUCB.0000011879.89900.1f
Viola, H., Mayer, R., and Jähne, E., Patent DD 144917, 1980.
Aghbash, K.O., Pesyan, N.N., Marandi, G., Dege, N., and Şahin, E., Res. Chem. Intermed., 2019, vol. 45, no. 9, p. 4543. https://doi.org/10.1007/s11164-019-03848-7
ACKNOWLEDGMENTS
The studies were carried out using the equipment of the Research and Education Center “Diagnostics of the Structure and Properties of Nanomaterials” and the Ecological Analytical Center of the Kuban State University.
Funding
This work was financially supported by the Russian Foundation for Basic Research and the Krasnodar Territory Administration (project no. 19-43-230007 r_a), as well as by the Ministry of Education and Science of the Russian Federation (topic 0795-2020-0031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 9, pp. 1340–1350 https://doi.org/10.31857/S0044460X21090055.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dotsenko, V.V., Guz, D.D., Tebiev, D.T. et al. Synthesis and Some Properties of New 5-Hydroxy-2-[(hetarylthio)methyl]-4H-pyran-4-ones. Russ J Gen Chem 91, 1629–1638 (2021). https://doi.org/10.1134/S107036322109005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322109005X