Abstract
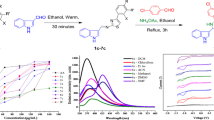
A simple one-pot synthetic method for novel S-alkenyl and propargyl derivatives of 5,5′-(1,4-phenylene)-bis(4H-1,2,4-triazole-3-thiol) and 5,5′,5″-(benzene-1,3,5-triyl)tris(4H-1,2,4-triazole-3-thiol) has been developed. Absorption and luminescence properties of the synthesized compounds have been estimated, and the fluorescence quantum yields have been determined.
Similar content being viewed by others
References
Andrews, B., Komathi, K., and Mohan, S., J. Chem. Sci., 2017, vol. 129, no. 3, p. 335. https://doi.org/10.1007/s12039-017-1228-z
Rajput, J.D., Bagul, S.D., Pete, U.D., Zade, C.M., Padhye, S.B., and Bendre, R.S., Mol. Diversity., 2018, vol. 22, no. 1, p. 225. https://doi.org/10.1007/s11030-017-9787-y
Gaber, M., El-Ghamry, H.A., Fathalla, S.K., and Mansour, M.A., Mater. Sci. Eng. C, 2018, vol. 83, p. 78. https://doi.org/10.1016/j.msec.2017.11.004
Gomha, S.M., Edrees, M.M., and El-Arab, E.E., J. Heterocycl. Chem., 2017, vol. 54, no. 1, p. 641. https://doi.org/10.1002/jhet.2636
Gyrdymova Yu.V., Demakova M.Ya., Shevchenko, O.G., Sudarikov, D.V., Frolova, L.L., Rubtsova, S.A., and Kuchin, A.V., Chem. Nat. Compd., 2017, vol. 53, no. 5, p. 895. https://doi.org/10.1007/s10600-017-2150-9
Kayumova, R.R., Muslukhov, R.R., Abdullin, M.F., Klen, E.E., Khaliullin, F.A., Magadeeva, G.F., Mamykin, A.V., and Khursan, S.L., Chem. Heterocycl. Compd., 2014, vol. 50, no. 7, p. 979. https://doi.org/10.1007/s10593-014-1553-9
Miao, S.-B., Li, Z.-H., Ji, B.-M., Deng, D.-S., Xu, C.-Y., and Zhou, L., J. Cluster Sci., 2014, vol. 25, no. 4, p. 1137. https://doi.org/10.1007/s10876-014-0695-3
Bushuev, M.B., Gatilov Yu.V., Krivopalov, V.P., and Shkurko, O.P., Inorg. Chim. Acta, 2015, vol. 425, p. 182. https://doi.org/10.1016/j.ica.2014.10.017
Tomma, J.H., Roúil, I.H., and Al-Dujaili, A.H., Mol. Cryst. Liq. Cryst., 2009, vol. 501, no. 1, p. 3. https://doi.org/10.1080/15421400802697160
Datoussaid, Y., Othman, A.A., and Kirsch, G.S., Afr. J. Chem., 2012, vol. 65, p. 30.
Darehkordi, A. and Ghazi, S., J. Chem., 2013, vol. 2013, p. 857956. https://doi.org/10.1155/2013/857956
Mobinikhaledi, A., Foroughifar, N., and Rafiee, A., Heterocycl. Commun., 2013, vol., 19, no. 4, p. 265. https://doi.org/10.1515/hc-2013-0035
Foroughifar, N., Mobinikhaledi, A., and Rafiee, A., J. Chem. Res., 2014, vol. 38, no. 2, p. 111. https://doi.org/10.3184/174751914X13896291543361
Zhang, W., Chai, Y., Li, K., Chen, Y., Yan, D., and Guo, D., Luminescence, 2014, vol. 29, no. 8, p. 1113. https://doi.org/10.1002/bio.2668
Bulut, N., Kocyigit, U.M., Gecibesler, I.H., Dastan, T., Karci, H., Taslimi, P., Dastan, S.D., Gulcin, I., and Cetin, A., J. Biochem. Mol. Toxicol., 2018, vol. 32, no. 1, p. e22006. https://doi.org/10.1002/jbt.22006
Uygun, Y., Bayrak, H., and Özkan, H., Turk. J. Chem., 2013, vol. 37, no. 5, p. 812. https://doi.org/10.3906/kim-1212-66
Il’inykh, E.S. and Kim, D.G., Chem. Heterocycl. Compd., 2011, vol. 47, no. 5, p. 636. https://doi.org/10.1007/s10593-011-0809-x
Il’inykh, E.S., Kim, D.G., Kodess, M.I., Matochkina, E.G., and Slepukhin, P.A., J. Fluorine Chem., 2013, vol. 149, p. 24. https://doi.org/10.1016/j.jfluchem.2013.01.025
Il’inykh, E.S. and Kim, D.G., Vestnik YUUrGU, Ser. Khim., 2015, vol. 7, no. 3. P., 19.
Il’inykh, E.S. and Kim, D.G., Chem. Heterocycl. Compd., 2011, vol. 47, no. 4, p. 524. https://doi.org/10.1007/s10593-011-0793-1
Eaton, D.F., Pure Appl. Chem., 1988, vol. 60, no. 7, p. 1107. https://doi.org/10.1351/pac198860071107
Williams, A.T.R., Winfield, S.A., and Miller, J.N., Analyst., 1983, vol. 108, p. 1067. https://doi.org/10.1039/AN9830801067
Nurmukhametov, R.N., Russ. Chem. Rev., 1967, vol. 36, no. 4, p. 693. https://doi.org/10.1070/RC1967v036n09ABEH001683
Funding
This work was performed with financial support from the Ministry of Science and Higher Education of the Russian Federation (grant no. 4.9665.2017/8.9) and within the framework of the state assignment (no. 075-00578-19-00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Il’inykh, E.S., Kim, D.G., Valova, M.S. et al. Synthesis and Optical Properties of New S-Derivatives of 5,5′-(1,4-Phenylene)bis(4H-1,2,4-triazole-3-thiol) and 5,5′,5″-(Benzene-1,3,5-triyl)tris(4H-1,2,4-triazole-3-thiol). Russ J Gen Chem 89, 2571–2576 (2019). https://doi.org/10.1134/S1070363219120375
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219120375