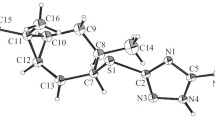
Thio-derivatives of triazole-substituted myrtanols were synthesized in 78–95% yields and were demonstrated to have membrane-protective and antioxidant properties based on their ability to inhibit H2O2-induced hemolysis of erythrocytes and LPO processes in brain lipids (in vitro).


Similar content being viewed by others
References
J.-Y. Yang, H.-W. Lee, and H.-S. Lee, Food Sci. Biotechnol., 24, 169 (2015).
G. Chawla, U. Kumar, S. Bawa, and J. Kumar, J. Enzyme Inhib. Med. Chem., 27, 658 (2012).
D. A. Ibrahim, Eur. J. Med. Chem., 44, 2776 (2009).
S. M. el-Khawass, M. A. Khalil, A. A. Hazzaa, H. A. Bassiouny, and N. F. Loutfy, Farmaco (Soc. Chim. Ital.), 44, 703 (1989).
T. Karabasanagouda, A. V. Adhikari, and N. S. Shetty, Eur. J. Med. Chem., 42, 521 (2007).
I. Ledeti, V. Bercean, A. Alexa, C. Soica, L.-M. Suta, C. Dehelean, C. Trandafirescu, D. Muntean, M. Licker, and A. Fulias, J. Chem., 1, 2015 (2015).
P. Zoumpoulakis, C. Camoutsis, G. Pairas, M. Sokovic, J. Glamoclija, C. Potamitis, and A. Pitsas, Bioorg. Med. Chem., 20, 1569 (2012).
M. Kritsanida, A. Mouroutsou, P. Marakos, N. Pouli, S. Papakonstantinou-Garoufalias, C. Pannecouque, M. Witvrouw, and E. De Clercq, Il Farmaco, 57, 253 (2002).
Z. Li, Y. Cao, P. Zhan, C. Pannecouque, J. Balzarini, E. Clercq, Y. Shen, and X. Liu, Med. Chem., 9, 968 (2013).
X.-Q. Deng, M.-X. Song, Y. Zheng, and Z.-S. Quan, Eur. J. Med. Chem., 73, 217 (2014).
A. Cetin and I. Gecibesler, J. Appl. Pharm. Sci., 120 (2015).
S.-F. Barbuceanu, D. Ilies, G. Saramet, V. Uivarosi, C. Draghici, and V. Radulescu, Int. J. Mol. Sci., 15, 10908 (2014).
L. Savegnago, M. do Sacramento, L. M. P. Brod, M. G. Fronza, N. Seus, E. J. Lenardao, M. W. Paixao, and D. Alves, RSC Adv., 6, 8021 (2016).
D. Hussain, Orient. J. Chem., 32, 539 (2016).
A. A. Hameed and F. Hassan, Int. J. Appl. Sci. Technol., 4, No. 2, 202 (2014).
R. Nimal, S. Aftab, U. A. Rana, A. Lashin, S. U.-D. Khan, S. Ali, H.-B. Kraatz, and A. Shah, J. Electrochem. Soc., 163, H871 (2016).
L. Nikitina, N. Artemova, and V. Startseva, Natural and Thiomodified Monoterpenoids: Synthesis and Biological Activity of Thioterpenoids [in Russian], LAP Lambert Academic Publishing, 2012.
E. V. Buravlev, I. Y. Chukicheva, O. V. Sukrusheva, O. G. Shevchenko, and A. V. Kutchin, Russ. Chem. Bull., 64, 1406 (2015).
E. S. Izmest′ev, D. V. Sudarikov, O. G. Shevchenko, S. A. Rubtsova, and A. V. Kutchin, Russ. J. Bioorg. Chem., 41, 77 (2015).
A. W. Snow and E. E. Foos, Synthesis, 0509 (2003).
J. K. Shneine and Y. H. Alaraji, Int. J. Sci. Res. (IJSR), 5 (3), 1411 (2016).
O. G. Shevchenko and L. N. Shishkina, Usp. Sovrem. Biol., 134, 133 (2014).
C. I. Acker, R. Brandao, A. R. Rosario, and C. W. Nogueira, Environ. Toxicol. Pharmacol., 28, 280 (2009).
. J.-S. Kim, Food Nutr. Sci., 4, 177 (2013).
S. T. Stefanello, A. S. Prestes, T. Ogunmoyole, S. M. Salman, R. S. Schwab, C. R. Brender, L. Dornelles, J. B. T. Rocha, and F. A. A. Soares, Toxicology in Vitro, 27 (5), 1433 (2013).
G. Zweifel and H. C. Brown, J. Am. Chem. Soc., 86, 393 (1964).
M. J. P. Ferreira, V. Emerenciano, G. A. Linia, P. Romoff, P. A. Macari, and G. Rodrigues, Prog. Nucl. Magn. Res. Spectrosc., 33, 153 (1998).
A. Banach, J. Scianowski, and P. Ozimek, Phosphorus Sulfur Silicon Relat. Elem., 189, 274 (2014).
T. Asakawa and S. Matsushita, Lipids, 15, 137 (1980).
J. J. M. Van den Berg, J. A. F. Opden Kamp, B. H. Lubin, B. Roelofsen, and F. A. Kuypers, Free Radical Biol. Med., 12, 487 (1992).
Acknowledgment
The work was supported financially in part by the RFBR (Project 16-53-00171 Bel_a) using equipment at the Khimiya Center for Collective Use, Inst. Chem., Komi SC, UB, RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2017, pp. 762–766.
Rights and permissions
About this article
Cite this article
Gyrdymova, Y.V., Demakova, M.Y., Shevchenko, O.G. et al. Synthesis and Antioxidant Activity of Myrtanylthiotriazoles. Chem Nat Compd 53, 895–900 (2017). https://doi.org/10.1007/s10600-017-2150-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2150-9