Abstract
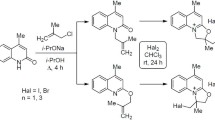
Alkylation of 4,8-dimethyl- and 4,6,8-trimethylquinolin-2(1H)-one with allyl bromide in the presence of a base proceeds at the oxygen and nitrogen atoms with the predominant formation of the O-isomer. The reaction of 2-allyloxyquinolines with halogens leads to the formation of oxazolo[3,2-a]quinolinium salts. Structure of 1-bromomethyl-5,7,9-trimethyl-1,2-dihydro[1,3]oxazolo[3,2-a]quinolinium iododibromide was established by single crystal X-ray diffraction method.
Similar content being viewed by others
References
Charushin, V.N., Nosova, E.V., Lipunova, G.N., and Chupakhin, O.N., Ftorkhinolony: sintez i primenenie (Fluo-roquinolones: synthesis and application), Moscow: FIZ-MATLIT, 2013. p. 24.
Aly, A.A., El-Sheref, E.M., Mourad, A.F.E., Bakheet, M.E., and Bräse, S., Mol. Diversity, 2019. https://doi.org/10.1007/s11030-019-09952-5
Joule, J.A. and Mills, K., Heterocyclic Chemistry, Wiley-Blackwell, 2010.
Gyul’budagyan, L.V., Aleksanyan, I.I., and Avetisyan, A.A., Arm. Khim. Zh., 1989, vol. 42, no. 10, p. 636.
Onysko, M.Yu. and Lendel, V.G., Chem. Heterocycl. Compd., 2007, vol. 43, no. 8, p. 1020. https://doi.org/10.1007/s10593-007-0159-x
Vershinina, E.A. and Kim, D.G., Vestn. YuUrGU, Ser. Khim., 2010, no. 31, p. 10.
Kim, D.G., Sashin, A.V., Kozlovskaya, V.A., and Andreeva, I.N., Chem. Heterocycl. Compd., 1996, vol. 32, no. 9, p. 1075. https://doi.org/10.1007/BF01164715
Ukrainets, I.V., Bereznyakova, N.L., Parshi-kov, V.A., and Turov, A.V., Chem. Heterocycl. Compd., 2007, vol. 43, no. 10, p. 1269. https://doi.org/10.1007/s10593-007-0193-8
Kim, D.G., Vershinina, E.A., Ovchinnikova, I.G., Slepu-khin, P.A., Ezhikova, M.A., and Kodess, M.I., Chem. Heterocycl. Compd., 2018, vol. 54, no. 10, p. 977. https://doi.org/10.1007/s10593-018-2374-z
Gorlov, M., Pettersson, H., Hagfeldt, A., and Kloo, L., Inorg. Chem., 2007, vol. 46, no. 9, p. 3566. https://doi.org/10.1021/ic062244b
Chernov’yants, M.S., Burykin, I.V., Berezova, P.N., and Starikova, Z.A., Mendeleev Commun., 2010, vol. 20, no. 3, p. 182. https://doi.org/10.1016/j.mencom.2010.05.021
Chernov’yants, M.S., Burykin, I.V., and Aleshina, N., Russ. J. Gen. Chem., 2008}, vol. 78, no. 7}, p. 1345. https://doi.org/10.1134/S1070363208070104
SMART and SAINT-Plus, Versions 5.0, Bruker AXS Inc., Madison, WI, USA, 1998.
SHELXTL/PC, Versions 5.10, Bruker AXS Inc., Madison, WI, USA, 1998.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J, Howard, J.A.K., and Puschmann, H., J. Appl. Cryst., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Russian Text © The Author(s), 2019, published in Zhurnal Obshchei Khimii, 2019, Vol. 89, No. 12, pp. 1829–1834.
Rights and permissions
About this article
Cite this article
Kim, D.G., Vershinina, E.A. & Sharutin, V.V. Synthesis and Halogenocyclization of 2-Allyloxyquinoline Derivatives. Russ J Gen Chem 89, 2353–2357 (2019). https://doi.org/10.1134/S1070363219120041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219120041